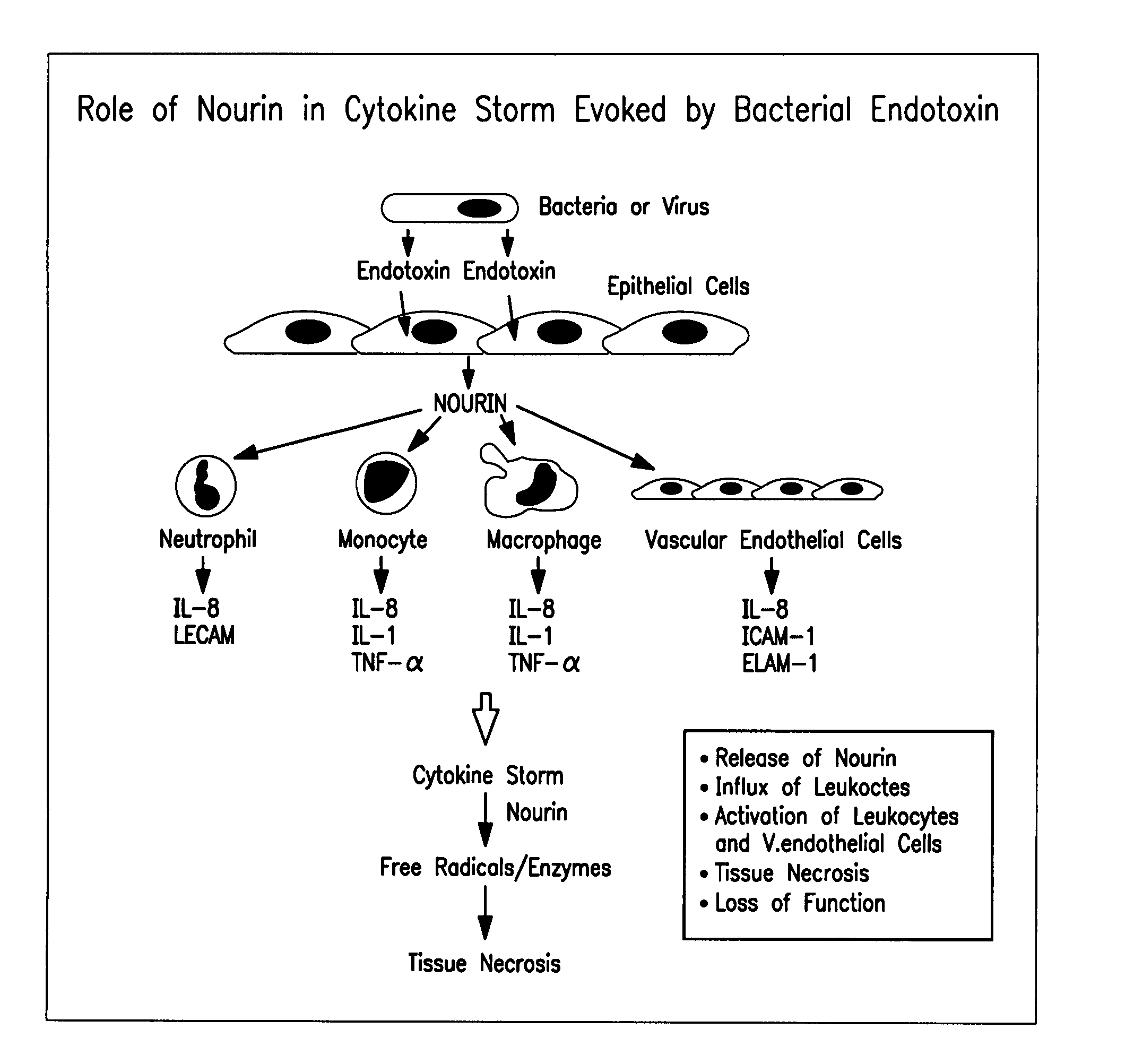
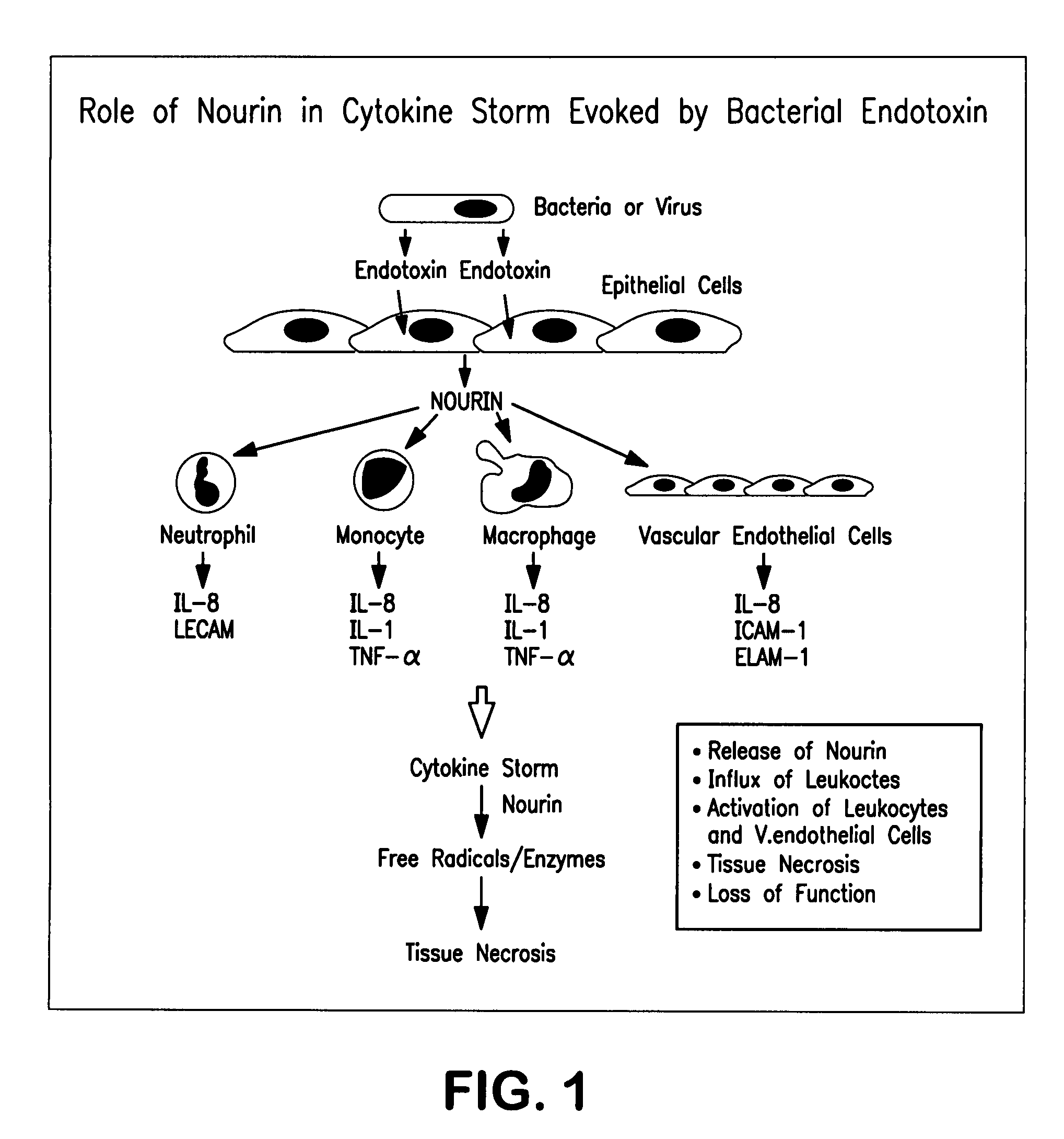
Methods to prevent and treat diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Release of Leukocyte Chemotactic Factors by Ischemic Spinal Cord Tissue
[0133] Three pigs were sacrificed and the spinal cord were immediately removed, cut, and incubated in Hank's Balance Salt Solution (HBSS) at room temperature (1 gm spinal cord / 2 ml HBSS). After 5, 10, 20, 40, 60, and 240 minutes, 100 microliter (ul) aliquots of supernatant solutions were collected and tested for the level of neutrophil chemotactic activity using human peripheral neutrophils as indicator cells. Samples collected from the 2 hour incubation were also processed on the size exclusion HPLC using the 1-300 KDa column. Modified Boyden chambers were used to test for the chemotactic activity in aliquot samples as undiluted, as well as diluted 1:5, and 1:25 in HBSS. The synthetic f-Met-Leu-Phe (10−9 Molar) was used as the positive control for 100% response, while HBSS was used as the negative control for random migration. Neutrophil migration was reported as chemotactic index of cell density (O.D. Units) u...
example 2
Alcohol-Induced Inhibitors of Leukocyte Chemotactic Factors
[0135] Studies have shown the association between acute and chronic ethanol intoxication and lowered resistance to infection in these patients (Feliu, 1977). Impairment of neutrophil chemotaxis due to the presence of serum inhibitors was suggested as a major mechanism for the increased susceptibility to infection (VanEpps, 1975). To date, tissue(s) releasing these serum inhibitors has not yet been identified.
[0136] Because the mucosal side of the gastric tissue is the first to be exposed to ingested ethanol and is exposed to the highest concentrations for a length of time, we tested the capability of gastric mucosa exposed to ethanol to release inhibitors of neutrophil chemotaxis. The mucosal surfaces of rabbit stomachs were incubated for 60 minutes with 0.01% ethanol (V / V) while the serosal sides were incubated with buffer. Results indicate that gastric tissue exposed to ethanol released inhibitors for neutrophil chemotax...
example 3
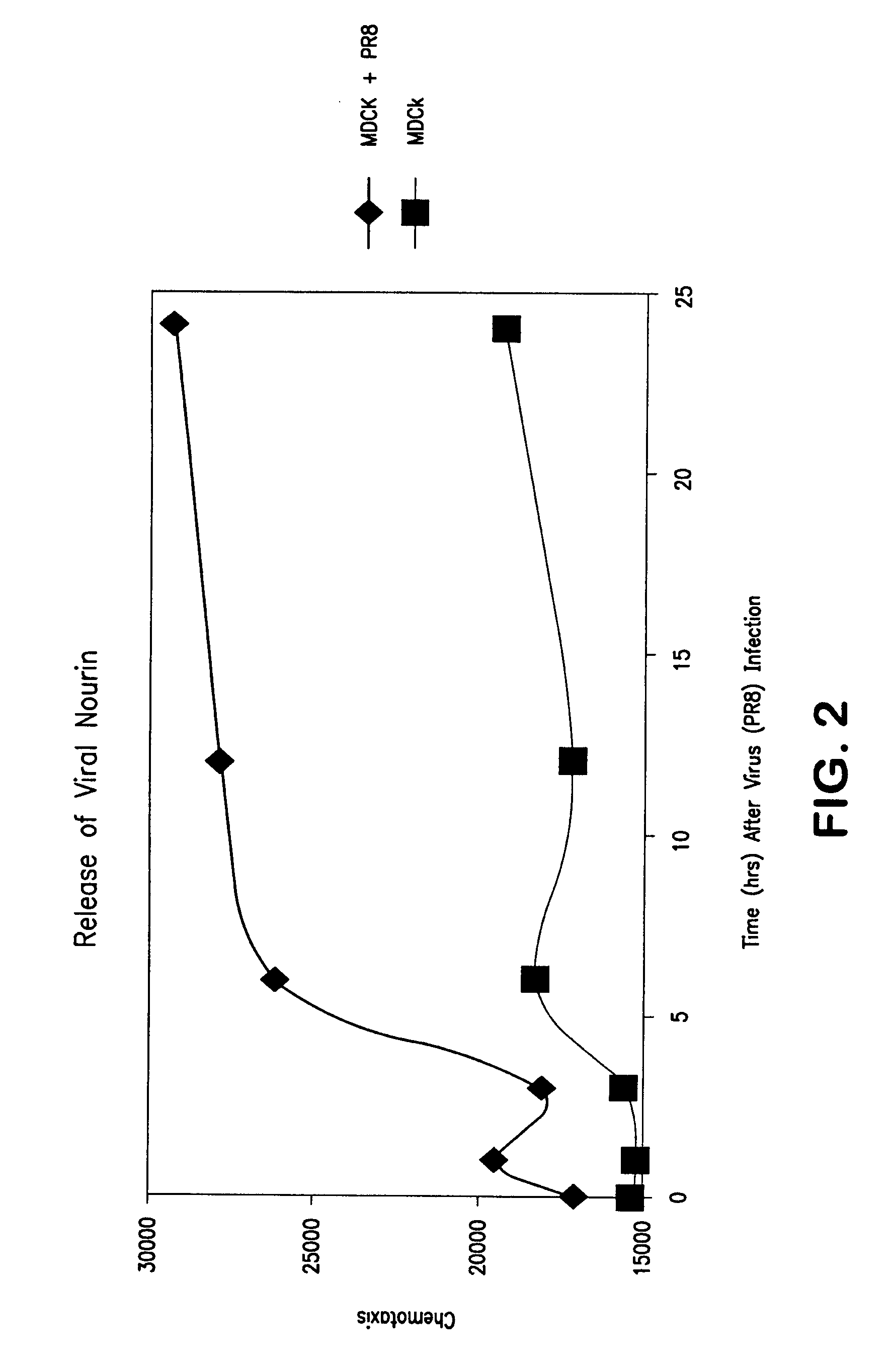
Leukocyte Chemotactic Factors Released by Epithelial Cells Infected with H1N1 Virus
[0139] We determined whether epithelial cells (Madin-Darby canine kidney cells-MDCK) grown in culture release LCFs in response to injury induced by laboratory-adapted influenza H1N1 virus. For these studies, MDCK cells were infected with the laboratory influenza virus H1N1 (PR8) for 1, 3, 6, 12, and 24 hours. Control cells were incubated with culture media only. At the various time points (1-24 hours), supernatant solutions were collected and tested for the presence and levels of chemotactic activity using standard modified chemotaxis chamber as a functional assay for chemotactic factors. Neutrophils isolated from human peripheral blood were used as the migratory cells.
[0140] As described in FIG. 2, significant chemotactic activity is detected in supernatant solutions collected from H1N1 infected cells compared to control cells grown in culture without the virus. High levels of chemotactic activity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body weight | aaaaa | aaaaa |
| Body weight | aaaaa | aaaaa |
| Energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com