Cultured Cell Sheet, Production Method and Tissue Repair Method Using Thereof
a production method and cell sheet technology, applied in the field of cultured cell sheet, a production method, and an applied tissue repair method thereof, can solve the problems of biological toxicity, limited site to be applied, toxicity to living organisms, etc., and achieve excellent flexibility, suppress air leakage, blood leakage or bodily fluid leakage, and high adhesiveness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0055]Hereinafter, the present invention will be explained in further detail based on the following Examples, which are not intended to limit the scope of the present invention in any way.
examples 1 and 2
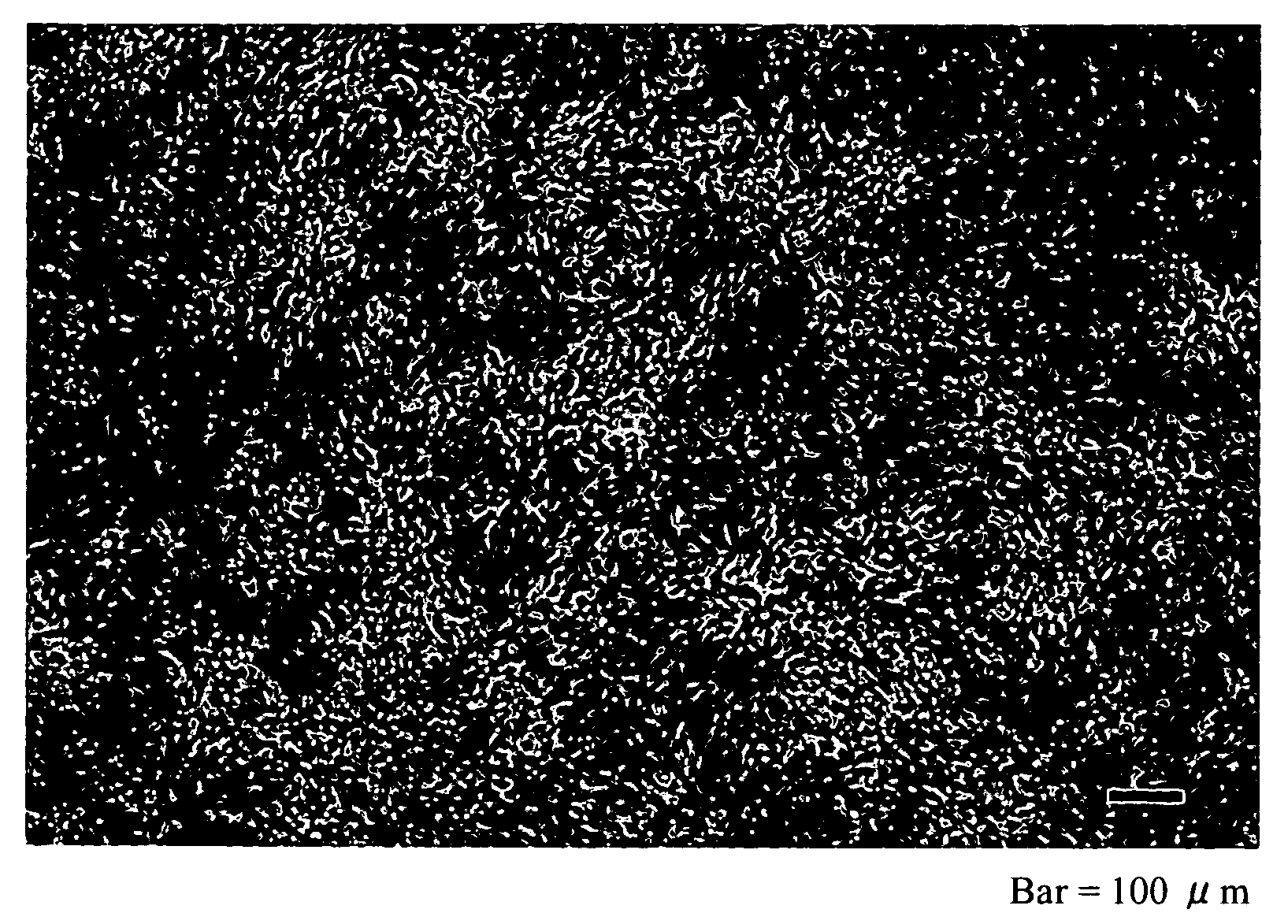
[0056]To a commercial culture dish with a diameter of 3.5 cm (Falcon 3001, manufactured by Becton Dickinson Labware), 0.07 ml of the solution of N-isopropyl acrylamide monomer dissolved in isopropyl alcohol at a concentration of 53% (Example 1) or 54% (Example 2) was applied. The culture dish was exposed to electron beams at an intensity of 0.25 MGy, and the N-isopropyl acrylamide polymer (PIPAAm) was immobilized on a surface of the culture dish. After irradiation, the culture dish was washed with ion-exchanged water to remove a residual monomer and the PIPAAm that did not bind to the culture dish, was then dried inside a clean bench, and sterilized by ethylene oxide gas, to obtain a cell culture support material coated with a temperature responsive polymer.
[0057]The amount of the temperature responsive polymer on the support surface was measured. As a result, it was found that the supports' surface was coated with the temperature responsive polymer in an amount of 1.7 μg / cm2 (Examp...
example 3
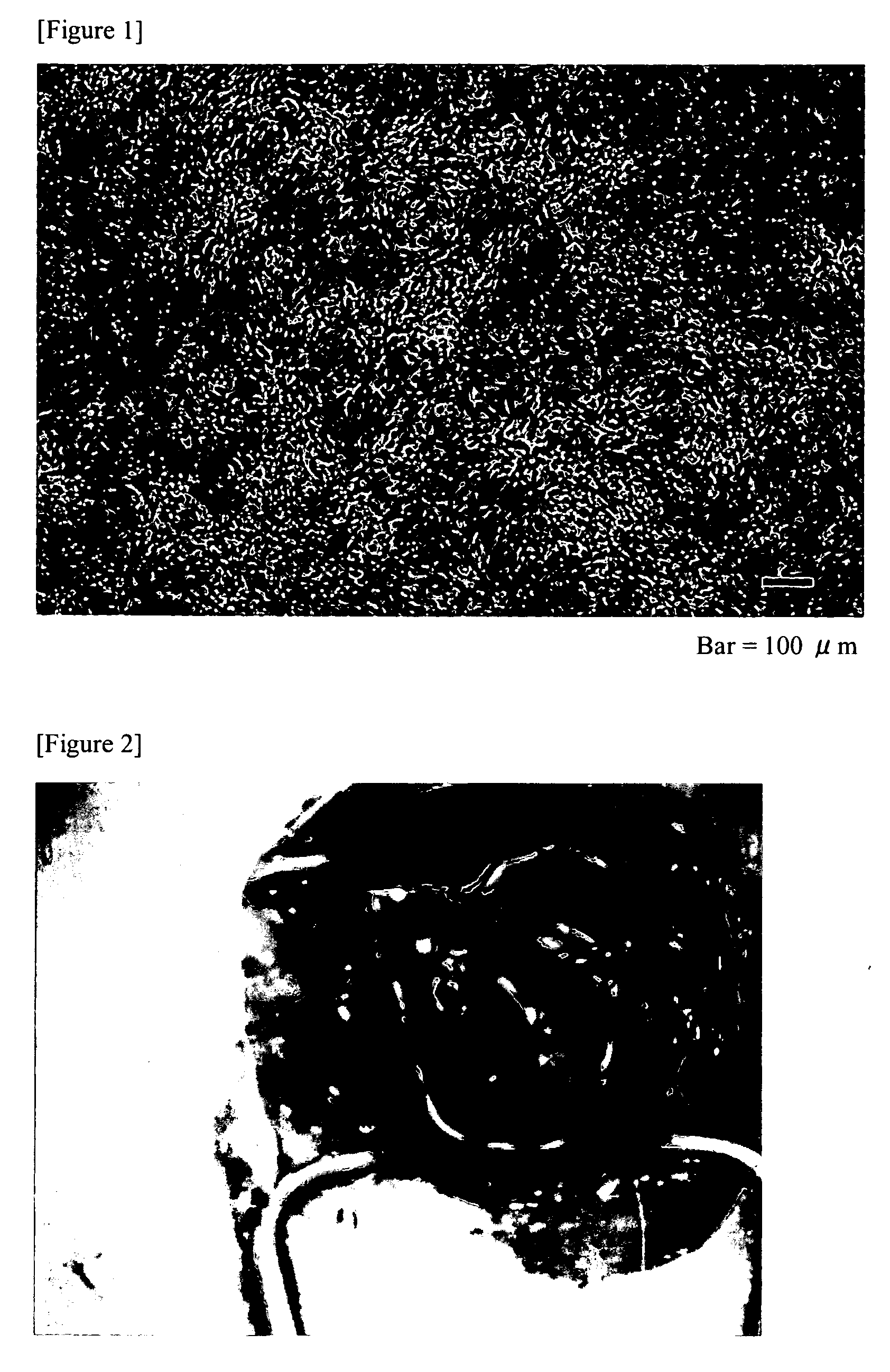
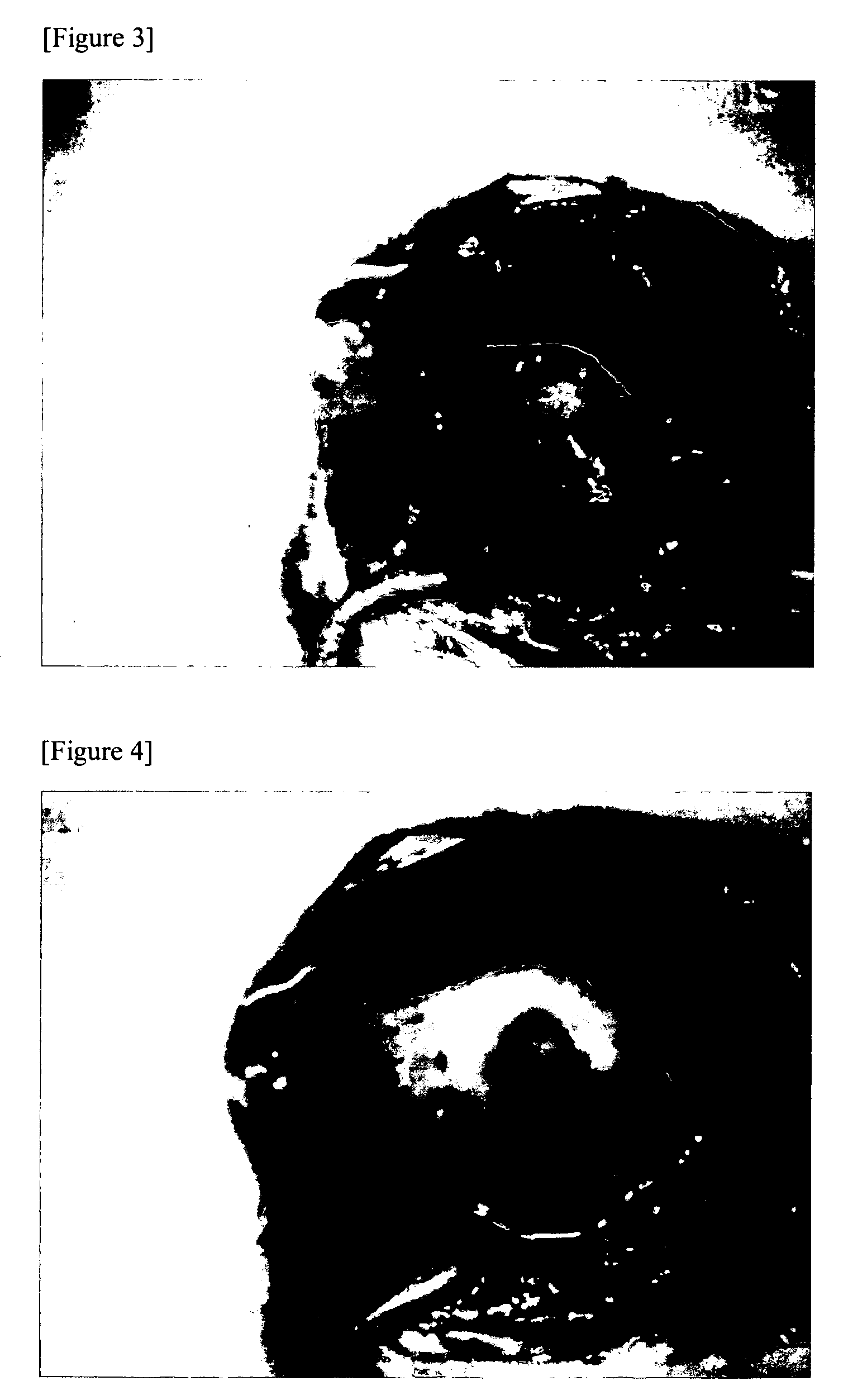
[0061]The study of this example is conducted in a similar manner to that of Example 2, except that 250 μM β-aminopropylnitrile was added to the culture medium, when cell passage was started on the cell culture support material, 3 days after the start of culture in Example 2. The cultured cell sheet obtained in the presence of β-aminopropylnitrile was mechanically flexible. The air leakage site was closed, and contracture of the covered portion of the cultured cell sheet was not found. Accordingly, the usefulness of a cultured cell sheet having flexibility as a tissue repair material could be confirmed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical solution temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| shrinkage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com