Water electrolysis system
a water electrolysis system and electrolysis technology, applied in the direction of electrode coatings, cell components, multiple component coatings, etc., can solve the problems of reducing the current efficiency of gas production, deteriorating the performance of the water electrolysis system in terms of purity, and extremely thin electrode catalyst layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
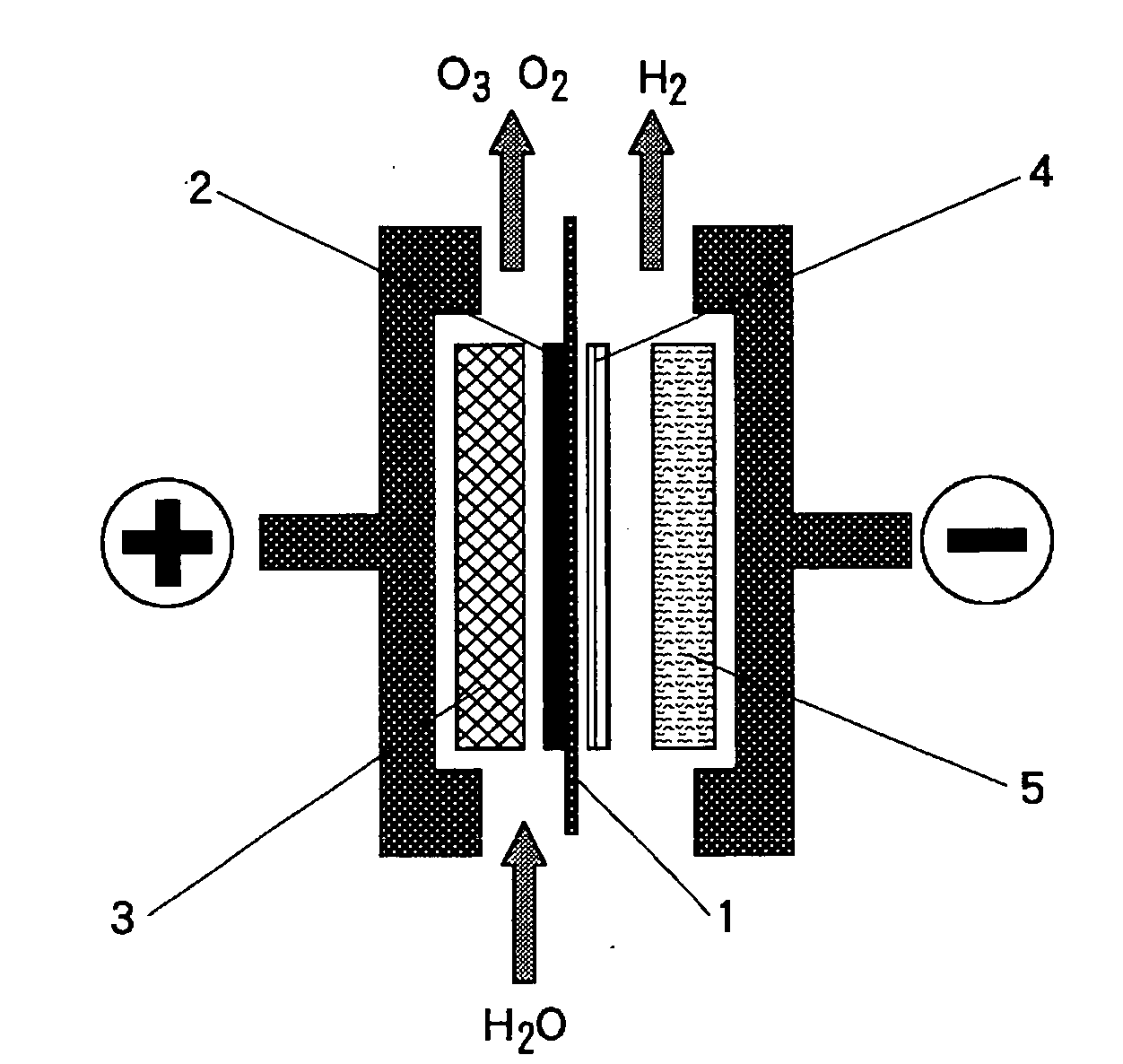
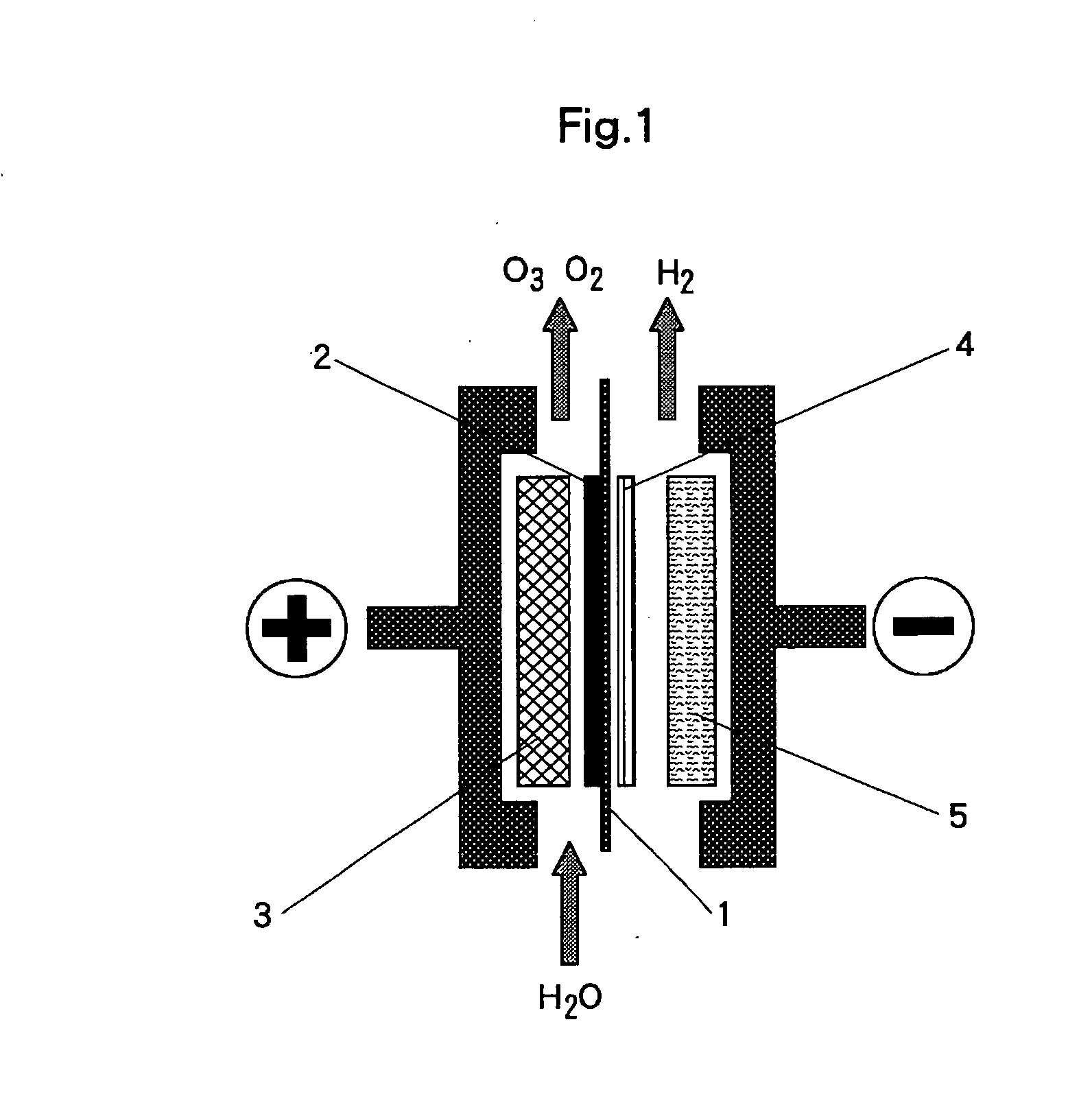
[0042]A sintered sheet of titanium fiber (manufactured by Tokyo Rope Mfg. Co., Ltd.), 1 mm thick, was washed with neutral detergent for degreasing and subject to pretreatment by acid pickling with 20 wt % hydrochloric acid solution for one minute at 50 degrees Celsius; then, on the said sintered sheet of titanium fiber, a coating comprising platinum-titanium-tantalum (25-60-15 mol %) was formed by the thermal decomposition method; and thus the anode current collector or the anode substrate with an underlayer on the surface is prepared.
[0043]Using said anode current collector or anode substrate as the anode, and 400 g / l of lead nitrate solution as electrolyte, electrolysis was performed for 60 minutes at 60 degrees Celsius at the current density of 1 A / dm2 to form a coating layer of β—lead dioxide, which is anode catalyst, on the anode current collector or the anode substrate surface.
[0044]A commercially available perfluorosulfonic acid type cation exchange membrane (Registered Trade...
example 2
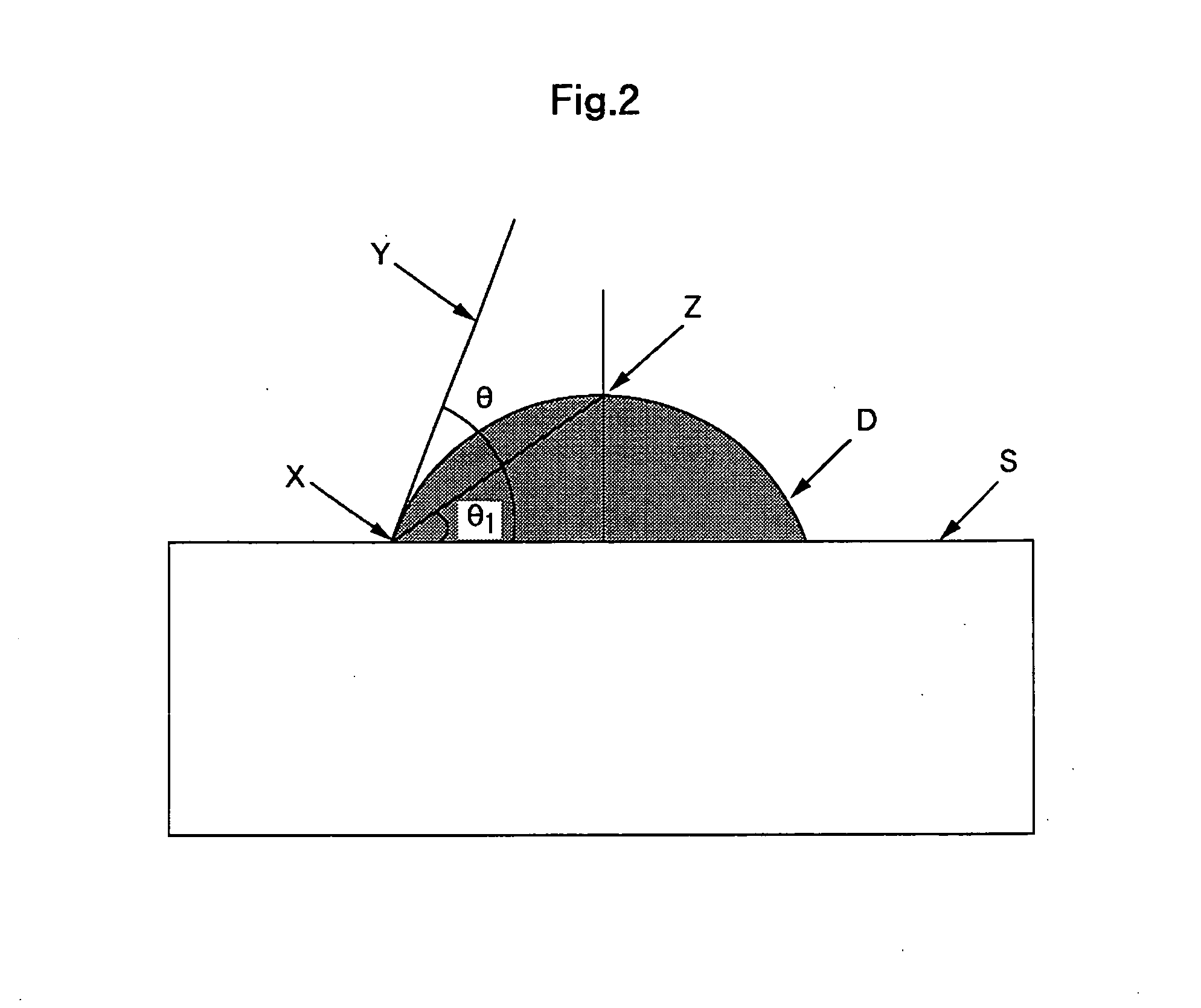
[0049]Cathode Sheet B was prepared in such way that an aqueous dispersion of PTFE dispersion and platinum-loaded carbon catalyst was applied with a brush on carbon paper (100 μm thick) surface, followed by drying, and this process was repeated three times to form Cathode Sheet B in porous structure comprising a carbon paper substrate of 110 μm thick. The contact angle with the catalyst coating surface of Cathode Sheet B was 95 degrees.
[0050]The electrolysis test was conducted as in Example 1, the results of which illustrated the concentration of ozone in the anode gas: 11.4 Vol. %, concentration of hydrogen gas in the anode gas: 0.10 Vol. %, and the cell voltage: 3.3 v.
example 3
[0051]A sintered sheet of titanium fiber, 1 mm thick, was washed with neutral detergent for degreasing and subject to pretreatment by acid pickling with 20 wt % hydrochloric acid solution for one minute at 50 degrees Celsius; then, on the said sintered sheet of titanium fiber, iridium dispersion prepared by dispersing iridium powder (under 200 mesh) in PTFE dispersion was applied with a brush until the final coating amount reached 250 g / m2 as iridium, followed by drying and thus, an iridium coated anode with a sintered sheet of titanium fiber was obtained.
[0052]The electrolysis test was conducted as in Example 1, wherein oxygen generated at the anode and hydrogen generated at the cathode and the results of which illustrated the concentration of hydrogen gas in the anode gas: 0.07 Vol. %, and the cell voltage: 2.5 v. For the cathode, Cathode Sheet A in porous structure was used, wherein the contact angle with the catalyst coating surface was 95 degrees
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com