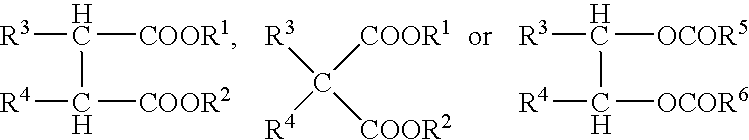
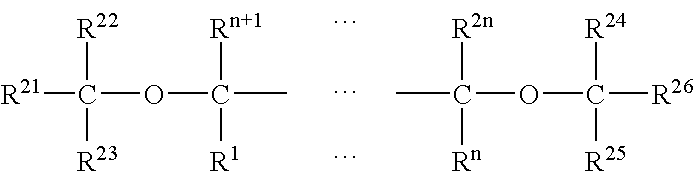
Catalyst for Olefin Polymerization and Polymerization Method Using the Same
a technology of olefin polymerization and catalyst, which is applied in the field of catalyst for olefin polymerization, can solve the problems of not having satisfactory performance in view of polymerization activity and stereoregularity, method also not having satisfactory performance in view of hydrogen response and stereoregularity upon polymerization, etc., to achieve high stereoregularity, low cost, and high fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[1] Preparation of Solid Titanium Catalyst Component
[0158][1-i] Preparation of Solid Component
[0159]A high-speed mixer with an internal volume of 2 liter (manufactured by Tokushu Kika Kogyo K. K.) was thoroughly purged with nitrogen gas, and 700 ml of purified kerosine, 10 g of commercial available magnesium chloride, 24.2 g of ethanol and 3 g of Emasol 320 (sorbitan distearate, a product of Kao-Atlas K. K.) were placed therein. While stirring the mixture, the temperature thereof was elevated and the mixture was stirred at 120° C. and 800 rpm for 30 minutes. Under the high-speed stirring, the resulting solution was transferred to a 2-liter glass flask (equipped with a stirrer) which was previously charged with 1 liter of purified kerosine cooled to −10° C. by using a Teflon (registered trademark) tube having an inner diameter of 5 mm. The resulting solid was collected by filtration and thoroughly washed with purified n-hexane to obtain a solid adduct in which ethanol coordinated to ...
example 2
[0166]The polymerization was carried out in the same manner as in Example 1, except that the process of [3] Main polymerization in Example 1 is changed as follows.
[3] Main Polymerization
[0167]A fully equipped, 5-liter autoclave was purged with hydrogen molecule, and into the autoclave, 1500 g of propylene was charged. After the temperature of propylene was elevated to 60° C., 50 ml of n-heptane, 0.41 mmol of triethylaluminum, 0.078 mmol of diethylaminotriethoxysilane, 0.0032 mmol of dicyclopentyldimethoxysilane, and 10 mg of the prepolymerized catalyst which had been prepared in the step of [2] Preparation of prepolymerized catalyst in Example 1 as a solid catalyst component were mixed therewith, and the mixture was injected to the autoclave with hydrogen molecule. The temperature of the autoclave was adjusted to 70° C., and during the polymerization, the pressure was maintained at 3.8 MPa / G with hydrogen molecule. The polymerization was carried out for 1 hour. After completion of t...
example 3
[0169]The polymerization was carried out in the same manner as in Example 1, except that the step of [2] Preparation of prepolymerized catalyst in Example 1 is changed as follows.
[2] Preparation of Prepolymerized Catalyst
[0170]Into a 1-liter autoclave which had been purged with nitrogen gas, 180 ml of n-heptane was charged, cooled to 0° C., and then into the autoclave, 9.0 mmol of triethylaluminum (TEA), 0.09 mmol of dicyclopentyldimethoxysilane, 9.95 g of propylene, and 0.9 mmol, in terms of titanium atoms, of the solid titanium catalyst component [A] which had been prepared in the step of [1] Preparation of solid catalyst component of Example 1 were charged. Then, the autoclave was tightly closed to perform the reaction at 20° C. for 1 hour under stirring. After completion of the polymerization, the reaction mixture was taken out under nitrogen gas atmosphere, and the liquid fraction was removed by decantation. The resultant fraction was three times washed with decane, and thus ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dipole moment | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com