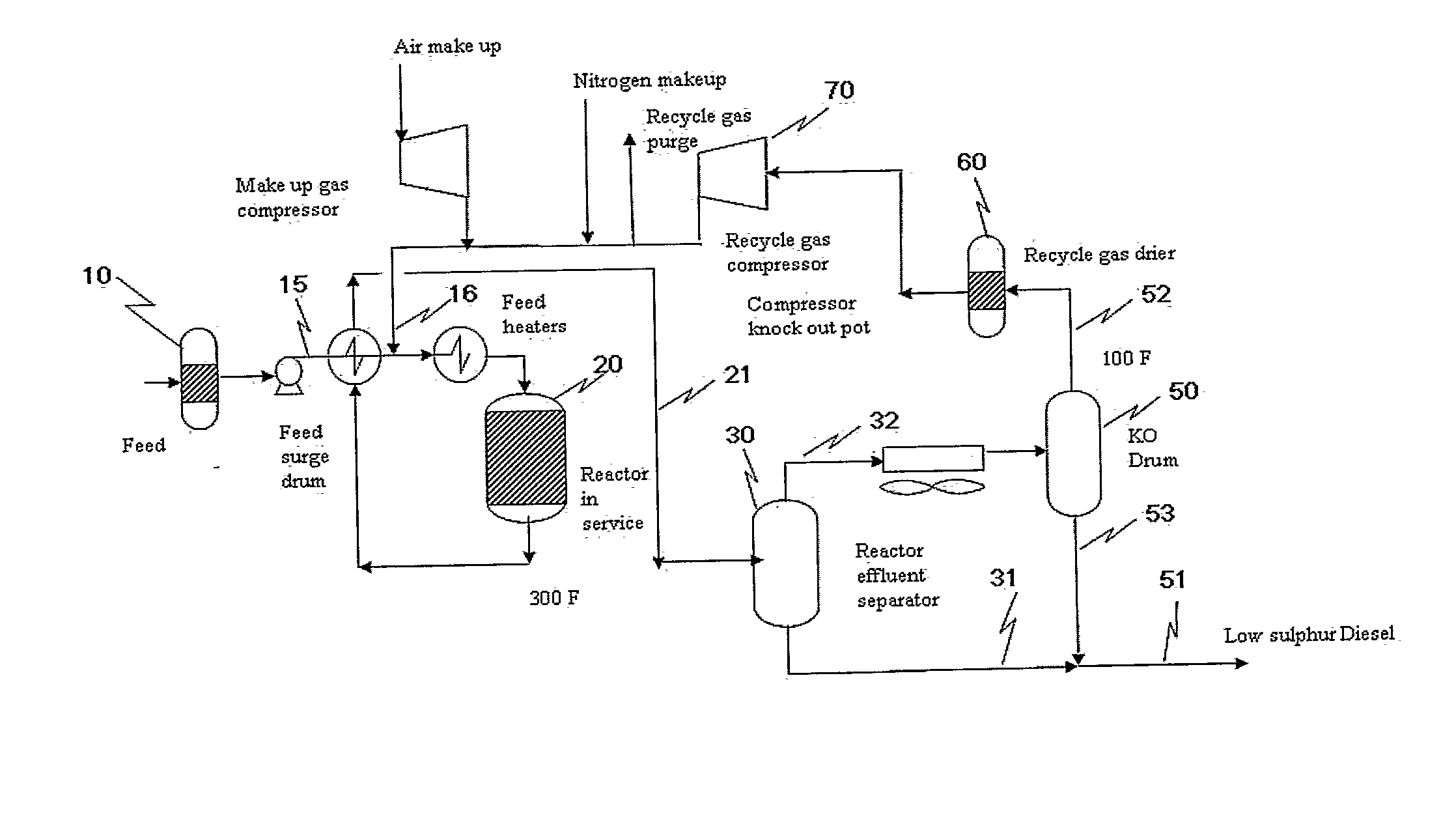
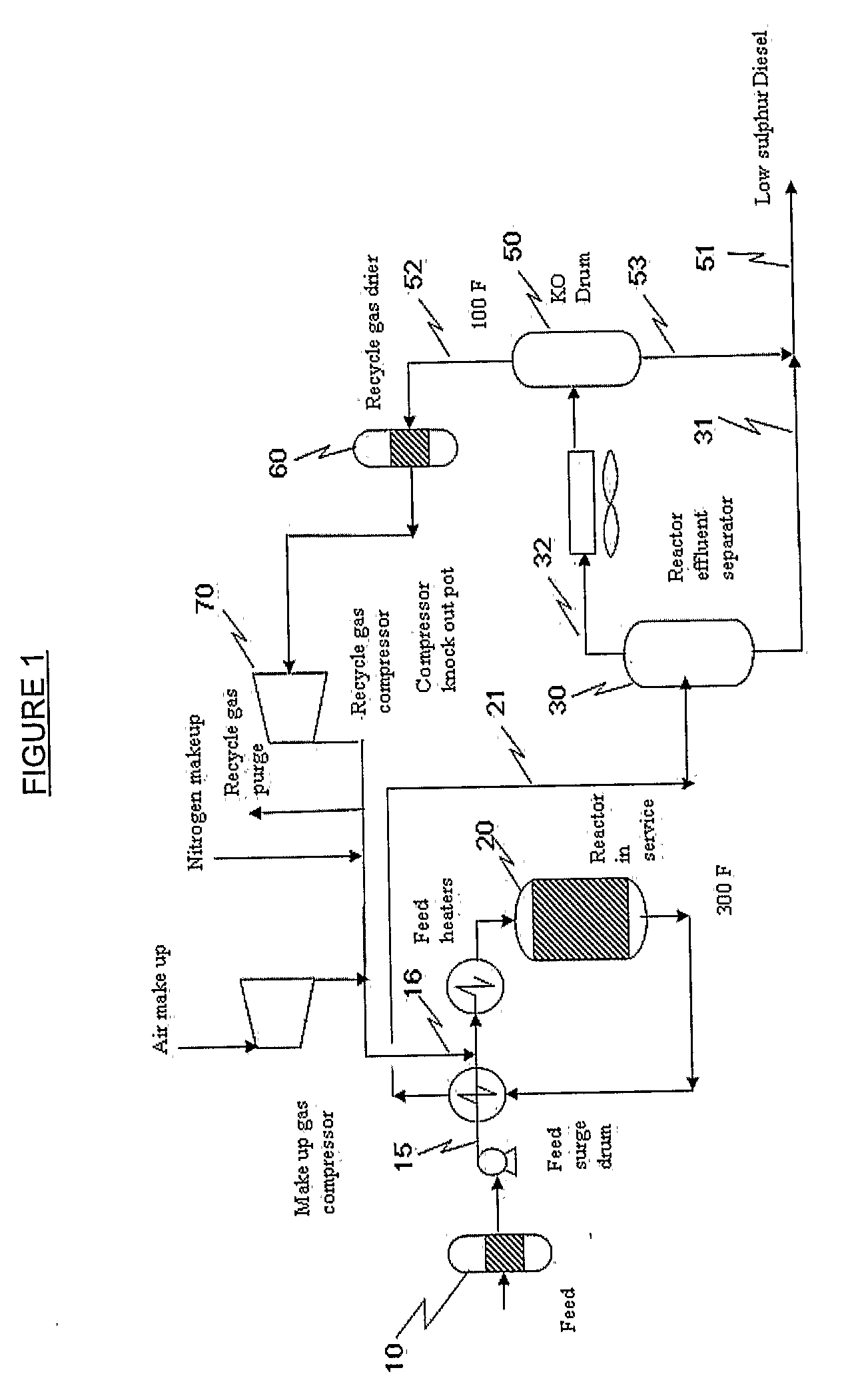
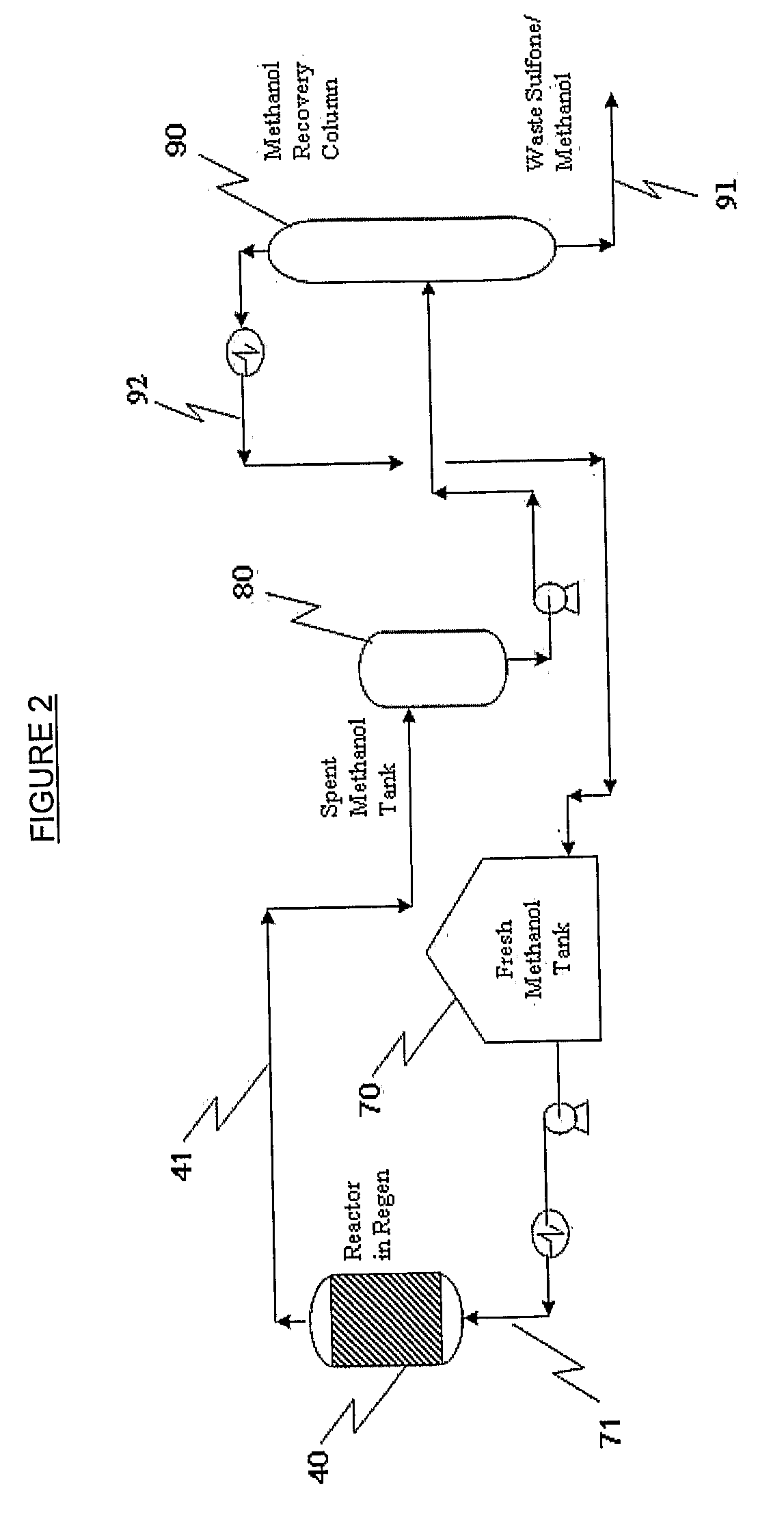
Oxidative Desulfurization Process
a desulfurization process and desulfurization technology, applied in the field of transportation-related fuels, can solve the problems of reducing the efficiency of the process,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0087]A titanium silicate used in the present invention as the oxidation catalyst and sulfone / sulfoxide adsorbent was prepared as follows: 350 grams of tetraethylorthoslicate were added to 500 grams of water. The tetraethylorthosilicate is immiscible with water and formed two layers with the top layer being the tetraethylorthosilicate: 139 grams of 10% in HCl was added which was soluble in the water layer. The two layers were heated with stirring to about 70° C. The initial reaction with the tetraethylorthosilicate formed a single layer which upon further heating formed a clear violet-gel. The gel was dried at room temperature to produce a solid. The solid was washed with 3 liters of water to reduce the amount of Cl. The catalyst was then dried overnight at 100° C. The solid can optionally be calcined at 500° C. for 4 hours. The yield of catalyst was 82 grams.
[0088]Three titania-silica catalysts prepared as described above were analyzed and were mostly amorphous), but Catalyst A and...
example 2
Experimental Equipment
[0089]Pilot-scale units were used to evaluate the performance of the catalyst with a 350 ppm-sulfur-containing diesel feed. The pilot-scale reactor consists of a 10.5 inch length 0.75 O.D.×0.438 inch I.D.×0.065 inch wall 316 Stainless Steel tubing. The reactor temperatures were maintained by three electrically heated sections of the reactor wall inside an insulated furnace box. The temperatures of these sections were controlled by a programmable computer with the use of single point thermocouples on each of the reactor wall sections. In addition, a 0.125 inch O.D. stainless steel thermowell that runs through the middle of the reactor from the top housed a multi-point thermocouple (three multi-point thermocouple with 2″ spacing) to monitor internal reaction temperature.
[0090]The pilot plant reactor consisted of a preheat zone filled with alumina chips, sieved to a Tyler screen mesh size of −20 +40 (USA Standard Testing Sieve by W. S. Tyler). The second and third...
example 3
[0103]In this example three separate deactivation runs were carried out.
[0104]Table 7 shows that essentially all of the sulfur was either deposited on the catalyst or recovered in the liquid product.
TABLE 7Three separate deactivation runs at 1.5 hr, 3 hr, and 4.5 hrto mass balance sulfur and determine S, N levels on catalyst.Run Description1.5 hr test3.0 hr test4.5 hr testCatalystCatalyst DescriptionTi silicateTi silicateTi silicateAge of Catalyst, hrslineout1.50lineout3.00lineout4.50Operating ConditionsReaction Temperature, ° F.325325325Pressure, psig200200200Gas Feed7% O2 / N27% O2 / N27% O2 / N2Gas Flow Rate, sccm250250250Feed Descriptionfinished dieselfinished dieselfinished dieselFeed LHSV1.01.01.0Analytical ResultsFeed sulfur, ppm-w335335335335335335Feed total nitrogen, ppm-w737373737373Product nitrogen, ppm-w552250165820Product sulfur, ppm-w286602767028490Product weight, g84.4317.1984.0232.7383.7750.96Absolute sulfur in product, g0.02410.00100.02320.00230.02380.0046Spent CatalystSu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com