Method and Apparatus for Measuring Protein Post-Translational Modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035]NAD+ kinase served as the acceptor chemical and as a controller chemical. Adenosine triphosphate served as the donor chemical.
[0036]Magnesium chloride and acetic acid served as controller chemicals.
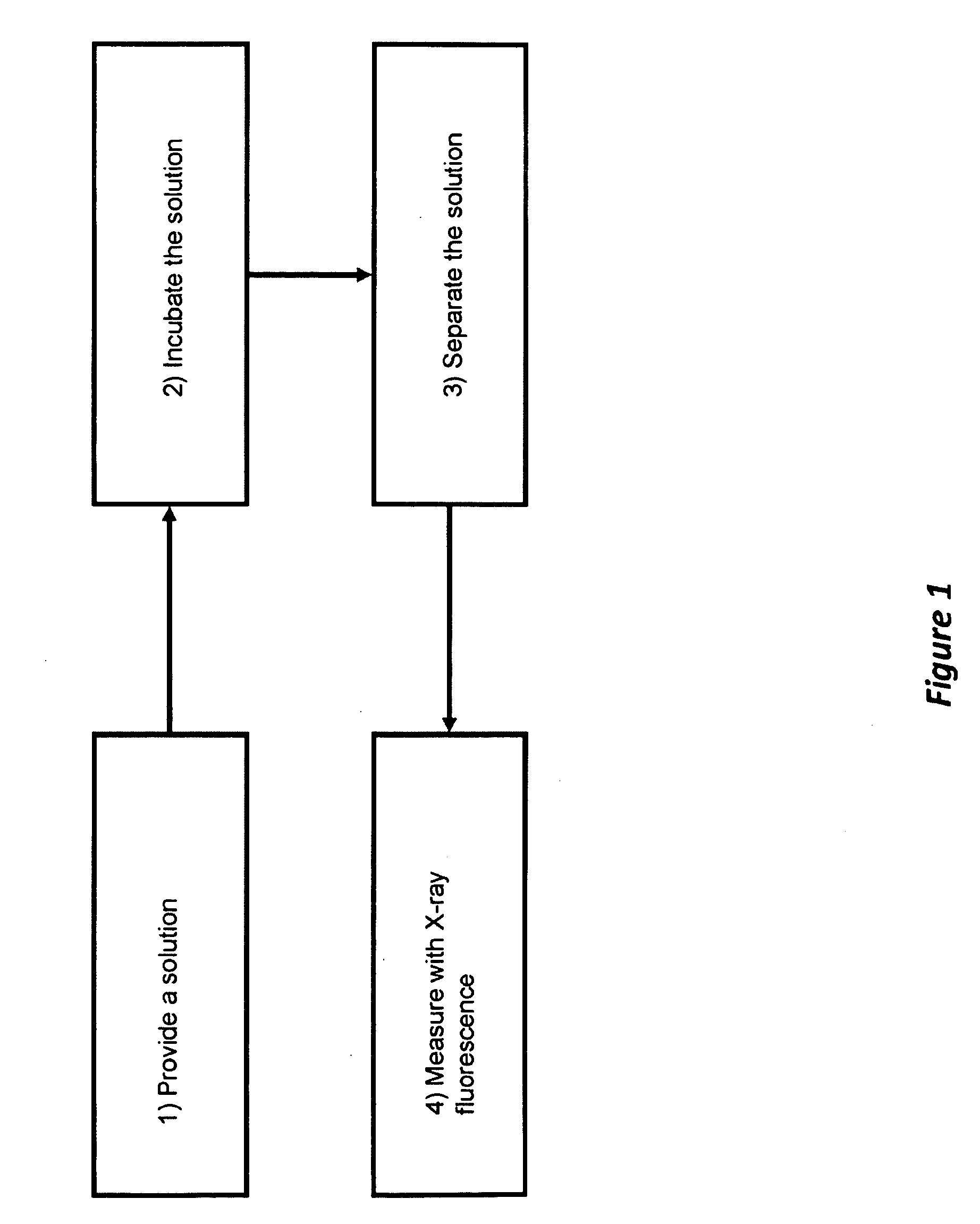
[0037]Phosphorylated NAD kinase served as the acceptor product. Adenosine diphosphate served as the donor product. A solution was prepared by combining 100 microliters of a solution of NAD kinase [880 micromolar] in water with a 200 microliters of a solution of adenosine triphosphate [5 millimolar], magnesium chloride [10 millimolar], tris(hydroxymethyl)aminomethane buffer [100 millimolar, pH 7.5] in water (FIG. 1, Box 1). The reaction was allowed to incubate for 10 seconds, after which 200 microliters of a solution of 5% acetic acid in water was added (FIG. 1, Box 2). The acceptor product was separated from the adenosine triphosphate and adenosine diphosphate using a Microcon 3000 molecular weight cut off (MWCO) ultrafilter, available from Millipore Corporate Headquarters, 290 Conc...
example 2
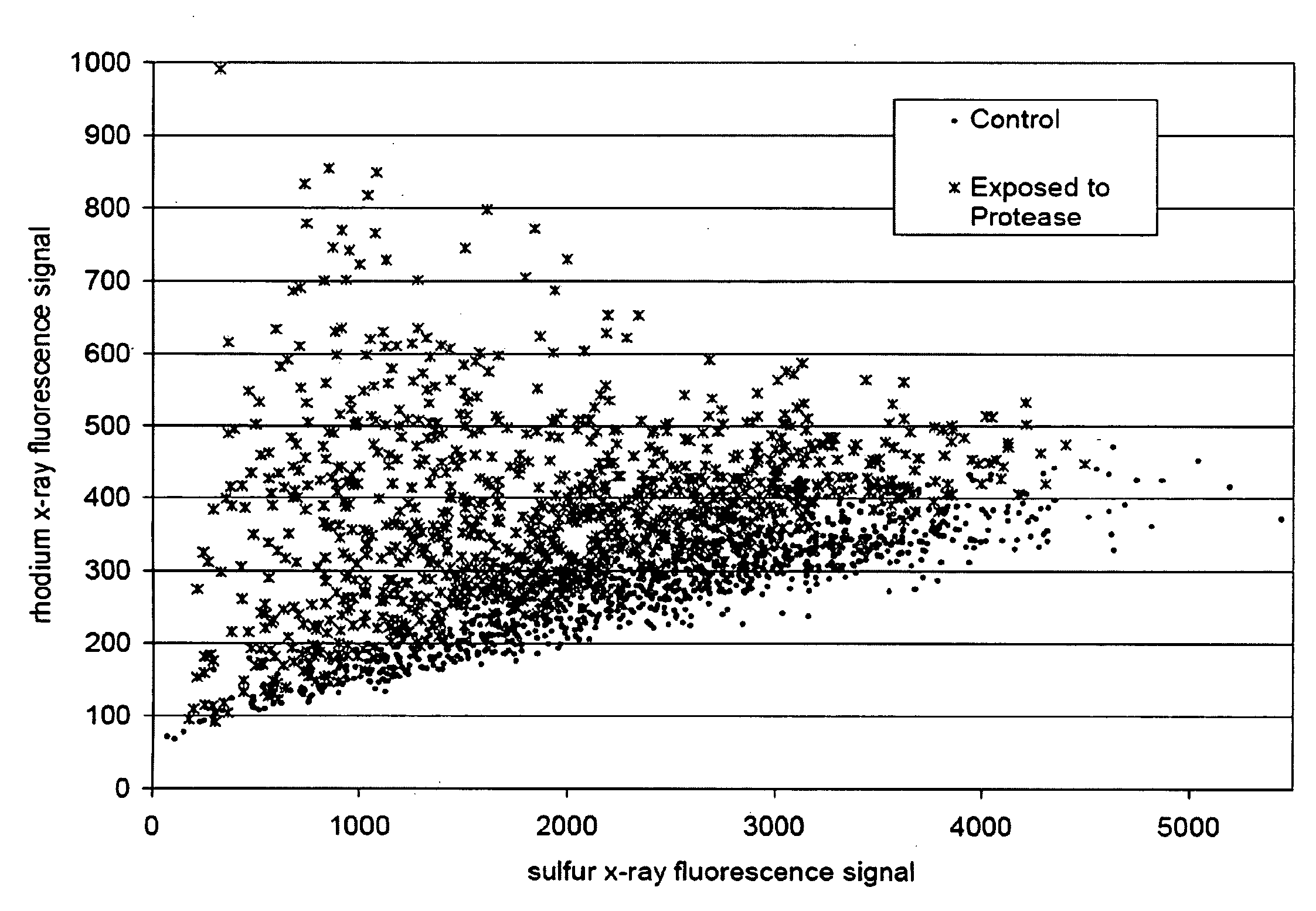
[0038]A set of peptides having the formula: polystyrene bead-LINKER-cysteine-1-xxx1-xxx2-xxx3-xxx4-cysteine2, where xxx1, xxx2, xxx3, and xxx4 are independently selected from the amino acids {alanine, arginine, asparagine, aspartic acid, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine} served as the donor chemical. “Polystyrene bead-LINKER” refers to AM resin such as available from Rapp Polymere GmbH, Emst-Simon-Str. 9, D 72072 Tübingen, Germany. Water served as the acceptor chemical. Trypsin and K2PtCl4 served as controller chemicals. The peptides having the formula: polystyrene bead-LINKER-cysteine1-xxx1-xxx2-xxx3-xxx4-CO2H served as the donor product, where xxx1, xxx2, xxx3, and xxx4 are independently selected from the amino acids {alanine, arginine, asparagine, aspartic acid, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, phenylalanine, proline, seri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com