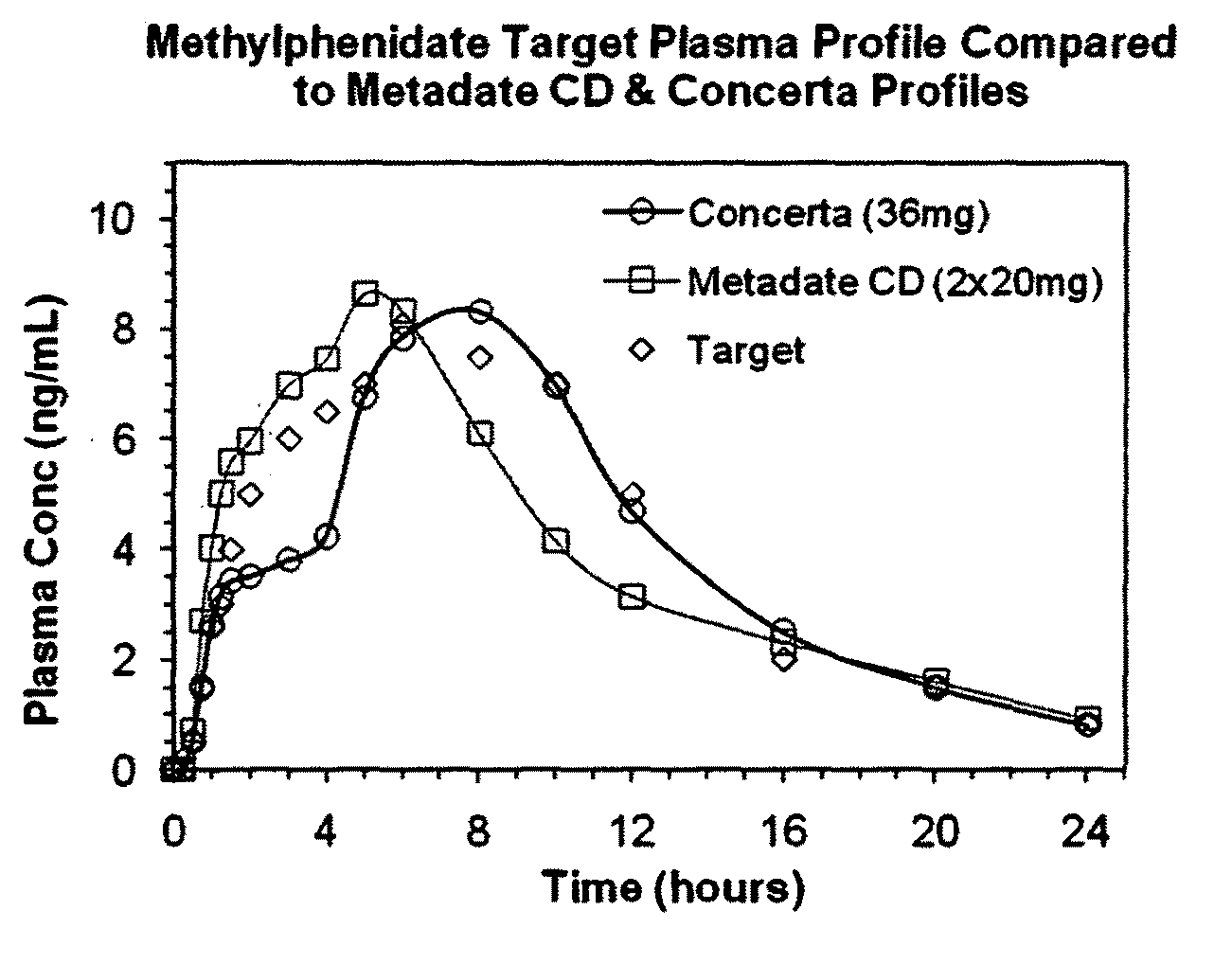
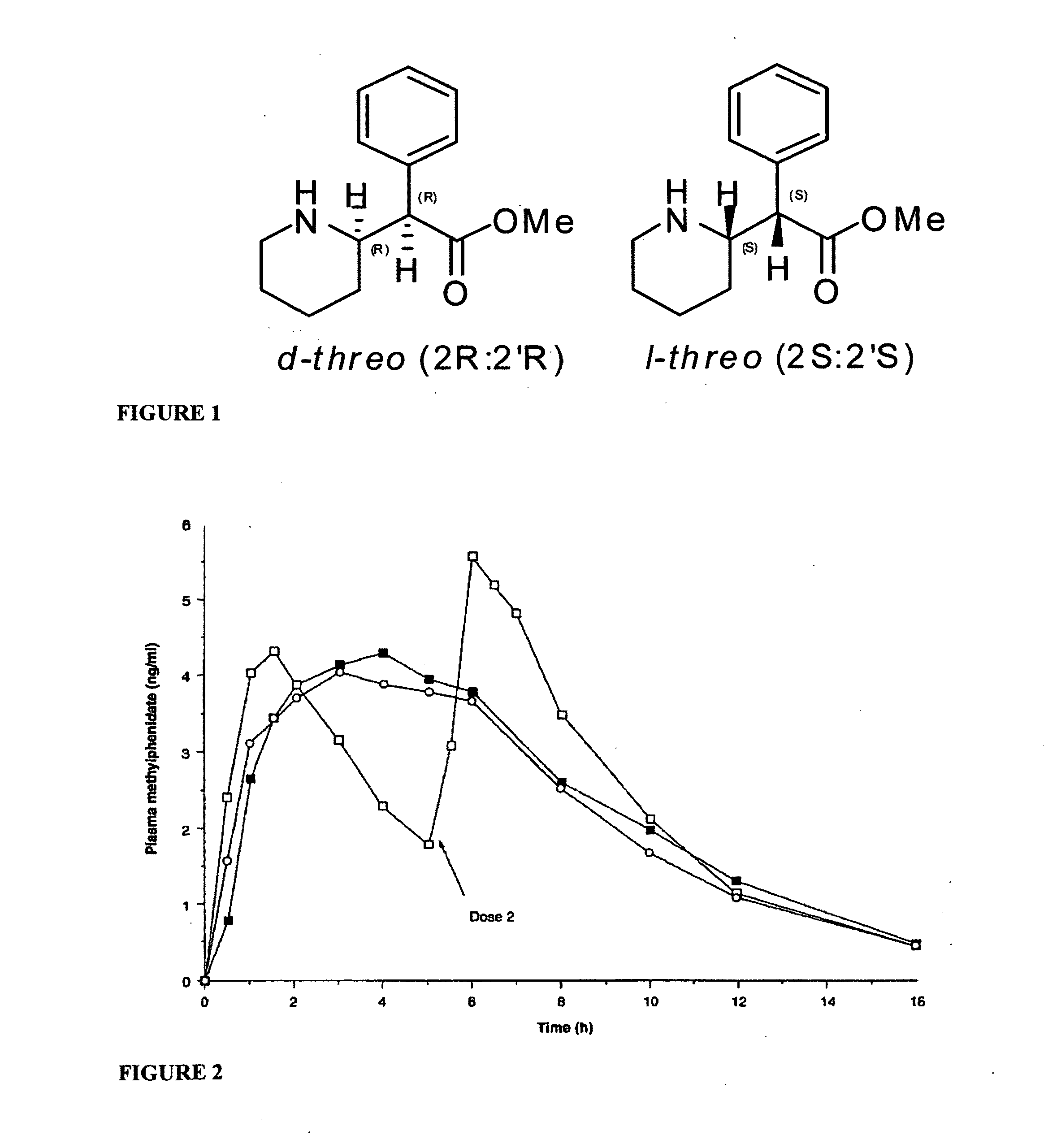
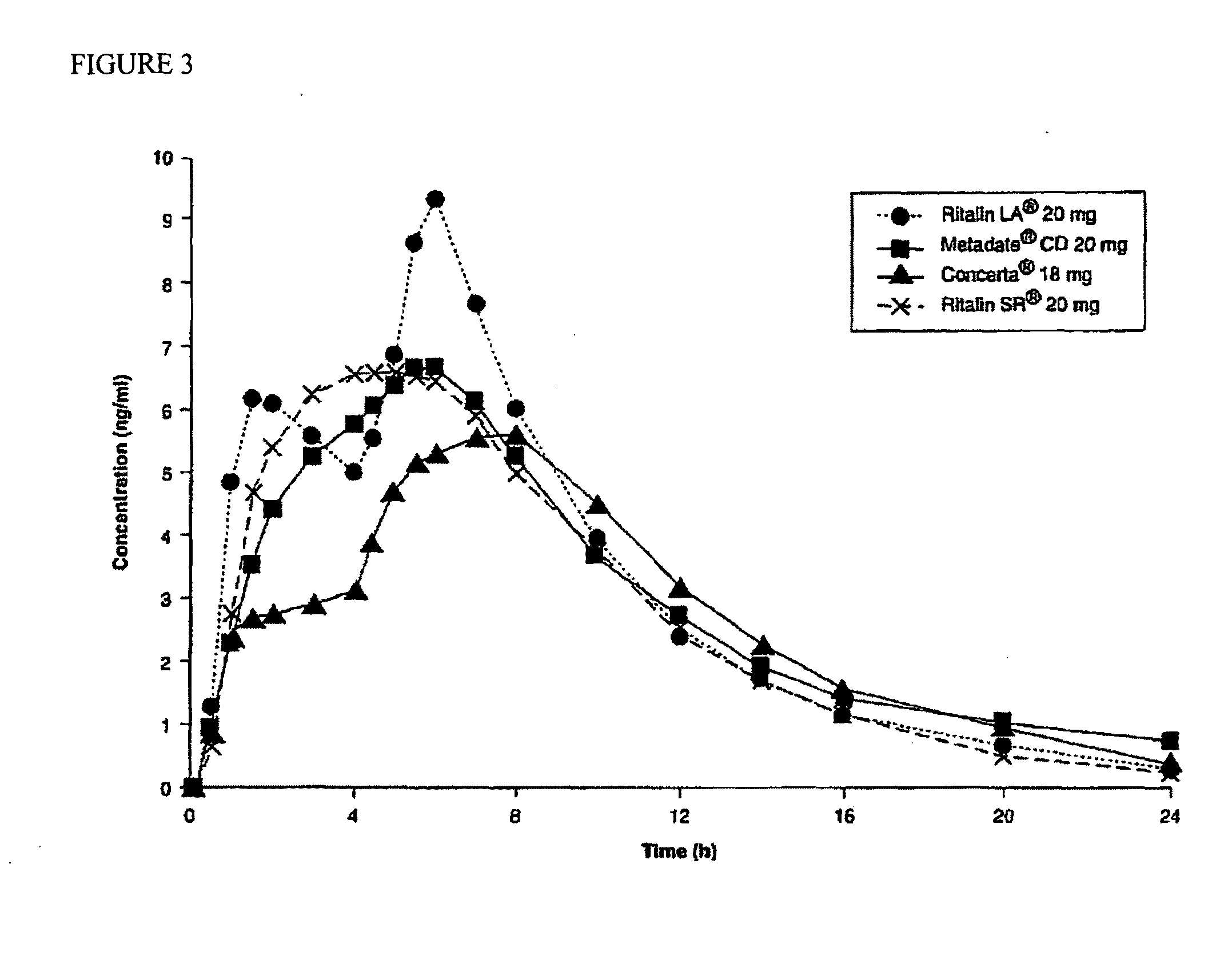
Oral pharmaceutical dosage forms
a technology of oral and syringe, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of increasing the surface area of the tablet, the inside of the tablet, and the difficulty in maintaining control of the drug formulation, so as to enhance the in vivo pharmacological performance, enhance the methylphenidate delivery kinetics, and enhance the safety features and/or abuse resistance properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Formulations
[0197](GMP Manufacturing Process)
[0198]A GMP manufacturing process for the dosage forms of the present invention was developed and carried out as follows. The following raw materials were used to create the formulations: methylphenidate (“MPH”); Isopropyl Myristate, NF (“IPM”); Colloidal silicon dioxide (Cabosil®, Cabot Corp) (“SiO2”); Butylated hydroxyl toluene, NF (“BHT”); Hydroxyethyl cellulose, NF (“HEC”); Sucrose Acetate Isobutyrate (Eastman Chemicals), (“SAIB”); Triacetin USP (“TA”); Cellulose Acetate Butyrate, grade 381-20 BP, ethanol washed (Eastman Chemicals) (“CAB”); Gelucire 50 / 13 (Gattefosse) (“GEL”); and Miglyol 812 (“MIG”). The formulations were filled into size #3 gelatin capsule shells. The specific details for the three different formulations produced using the GMP manufacturing processes of this Example 1 are disclosed below in Tables 1 and 2. The batch sizes were up to 500 g.
TABLE 1Formulation by Weight Percent (wt %)MPH1MPH2MPH3MPH11MPH...
example 2
Analysis of Formulations
[0200](In Vitro Dissolution Testing Procedures)
[0201]Two in vitro dissolution test methods were developed in order to assess the controlled release performance of abuse-resistant dosage forms produced according to the present invention such as the methylphenidate dosage forms recited herein. The first dissolution method (Method 1) was based upon USP Method A for delayed-release dosage forms and uses an USP dissolution apparatus Type 2 (without basket) with a two-stage media (an initial volume of 750 mL of 0.1N HCl acid as the dissolution medium, followed by adjustment to pH 6.8 by addition of 250 mL of sodium phosphate buffer after 2 hours). The two-stage media was selected to simulate the pH range over which a dosage form will release active agent during transit through the GI tract. Stainless steel coiled wire type 316 is used as a sinker to ensure that the dosage forms remain at the bottom of the dissolution vessel during release rate testing.
[0202]The se...
example 2a
[0208]The following in vitro dissolution test was carried out to characterize the in vitro release of abuse-resistant methylphenidate oral dosage forms across several different formulations.
[0209]The abuse-resistant methylphenidate oral dosage forms used in this Example 2a were prepared using the following raw materials: methylphenidate (“MPH”); Isopropyl Myristate, NF (“IPM”); Colloidal silicon dioxide (Cabosil®, Cabot Corp) (“SiO2”); Butylated hydroxyl toluene, NF (“BHT”); Hydroxyethyl cellulose, NF (“HEC”); Sucrose Acetate Isobutyrate (Eastman Chemicals), (“SAIB”); Triacetin USP (“TA”); Cellulose Acetate Butyrate, grade 381-20 BP, ethanol washed (Eastman Chemicals) (“CAB”); Gelucire 50 / 13 (Gattefosse) (“GEL”); and Miglyol 812 (“MIG”). The formulations were produced using the manufacturing process as described in Example 1 above, and then filled into size #3 gelatin capsule shells to produce the dosage forms that were used as Test Capsules. The details of the formulations and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com