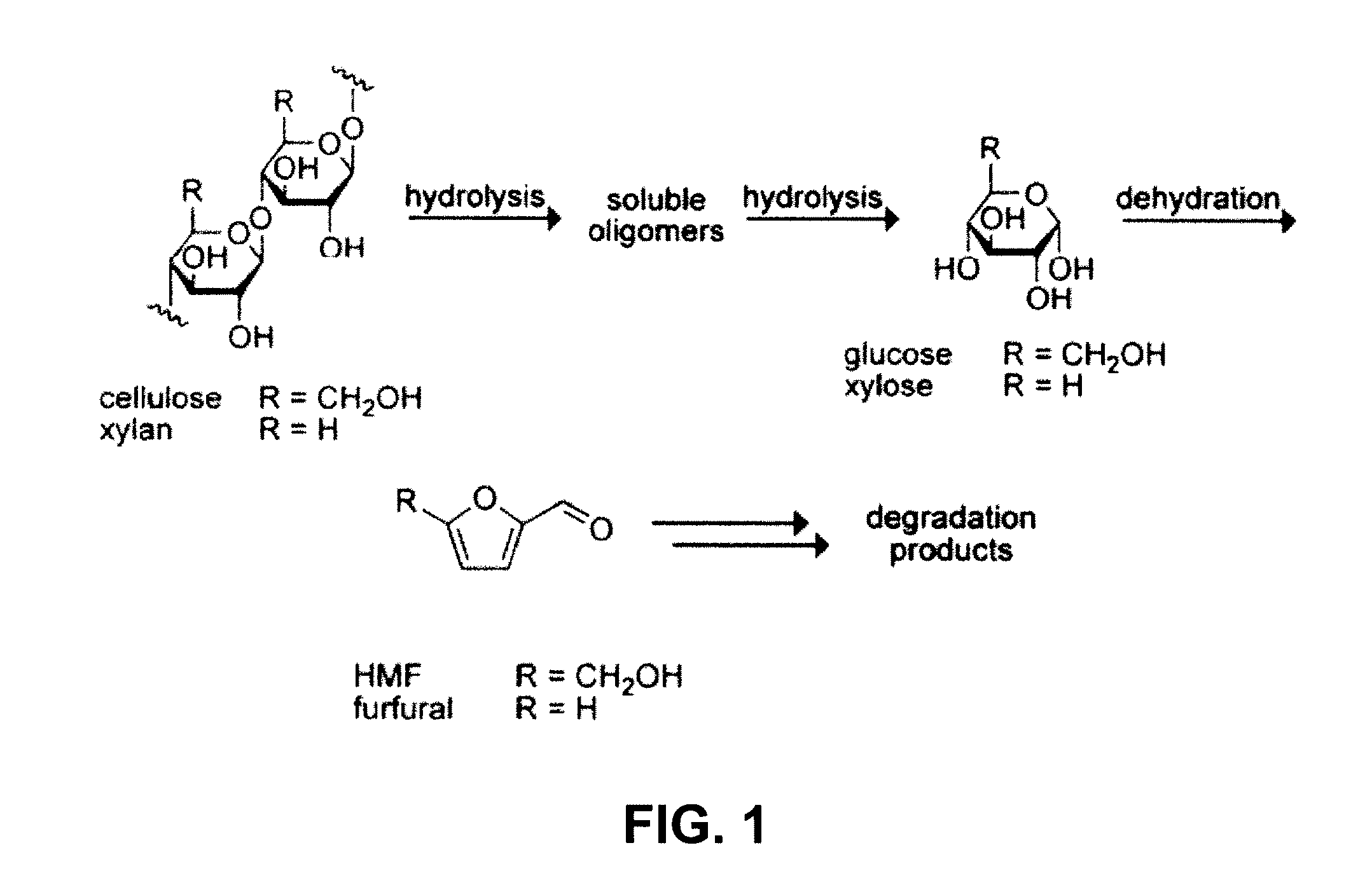
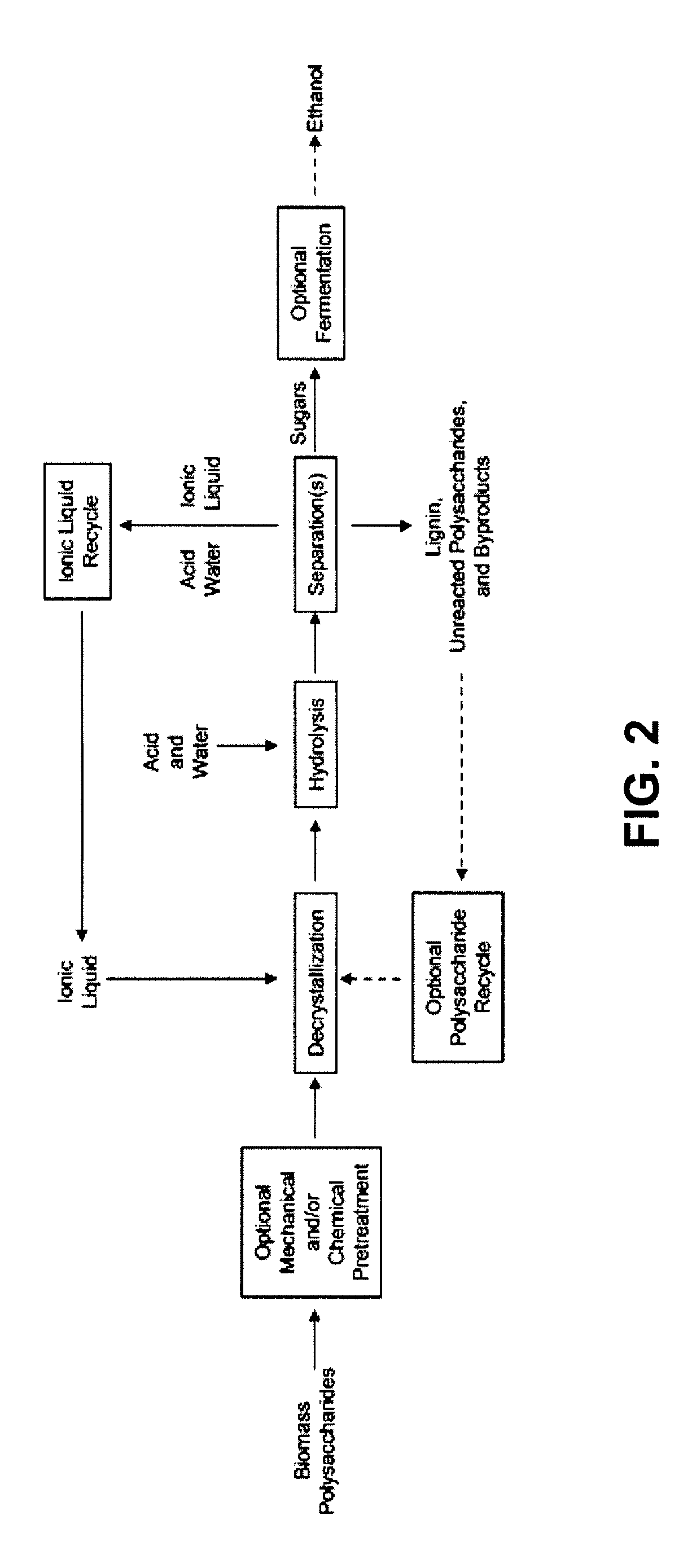
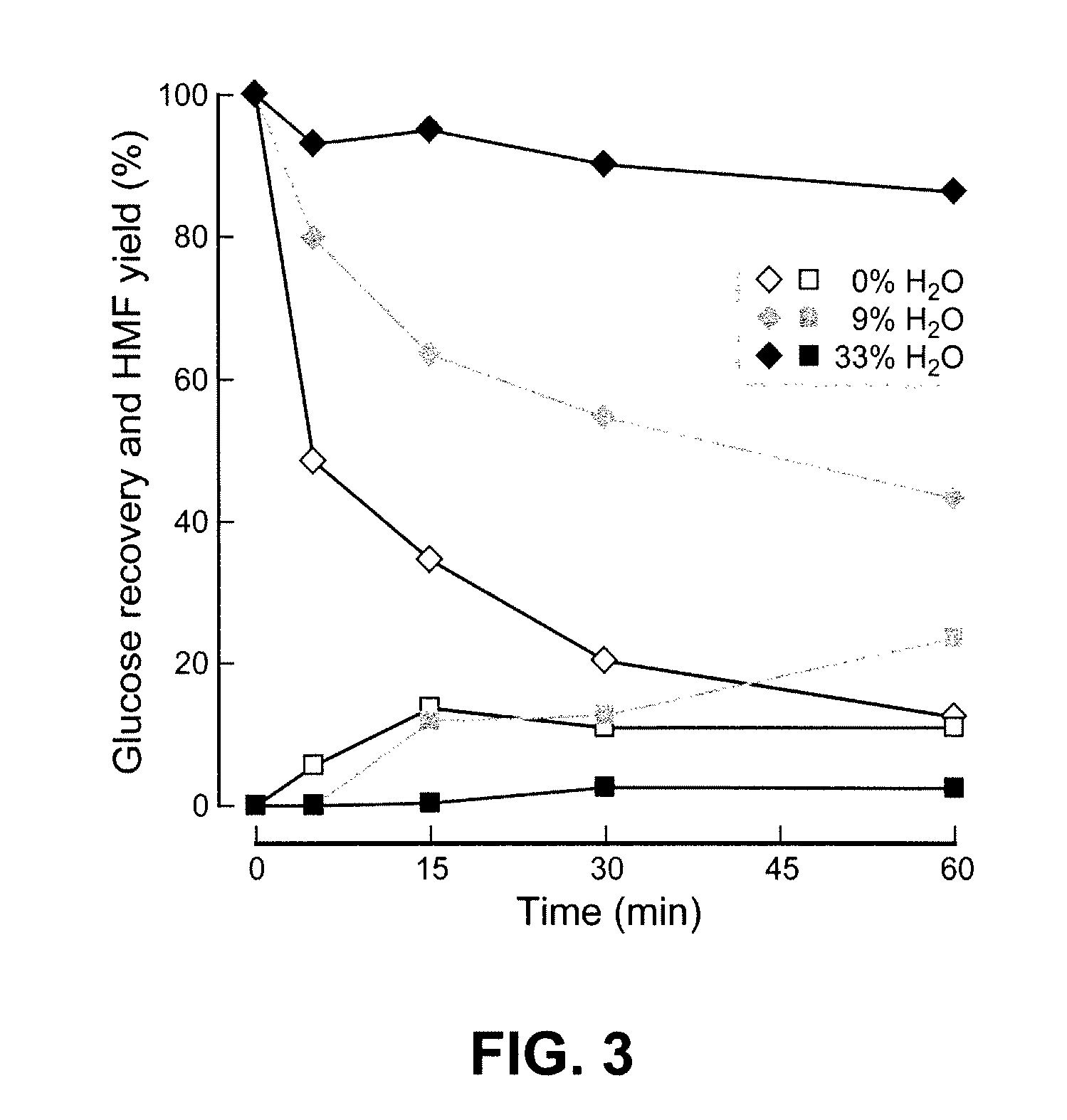
[0022]In the case of cellulose or other
polysaccharide, hydrolysis is continued to achieve a maximum yield of glucose, while minimizing the production of undesired
monosaccharide dehydration by-products, such as hydroxymethylfuran (HMF). In specific embodiments, the glucose yield from
cellulose hydrolysis is equal to or greater than 50%. In specific embodiments, the glucose yield from
cellulose hydrolysis is equal to or greater than 75%. In specific embodiments, the glucose yield from
cellulose hydrolysis is equal to or greater than 85%. In specific embodiments, the hydrolysis reaction is carried out for 1-10 hours, more preferably 1-5 hours, and more specifically 2-3 hours. In a specific embodiment, the reaction is carried out at a temperature
ranging from 100-110° C. for 1-4 hours and more specifically for 2-3 hours. The addition of water as described decreases the formation of undesired by-products of cellulose hydrolysis, specifically hydroxymethylfuran (HMF), which are believed at least in part to result from
monosaccharide dehydration. In specific embodiments, the yield of HMF in the hydrolysis is 10% or less. In specific embodiments, the yield of HMF in the hydrolysis is 5% or less. The
monosaccharide products of hydrolysis can be separated from ionic liquid and employed as a source of monosaccharide for any desired application. In a specific embodiment, the products of hydrolysis can be separated from ionic liquid and employed as a source of monosaccharide for growth of microorganisms and the production of
fermentation products, such as
ethanol. In an embodiment, the ionic liquid can be separated from the hydrolysis product, particularly by passage through an appropriate
ion exchange column and the ionic liquid can optionally be recycled for reuse.
[0029]In the processes herein, water is added after
acid catalyzed hydrolysis of cellulose is initiated to enhance glucose yields and minimize by-product generation, e.g., generation of HMF. The timing and amount of water addition is controlled to avoid cellulose
precipitation, increase glucose yield (or yield of glucose combined with other monosaccharide) and minimize undesired dehydration by-product, e.g., HMF, formation. It will be readily appreciated that the initial reaction mixture may contain incidental low levels of total water that are in the ionic liquid, in the cellulose or lignocellulose, or otherwise enter the reaction vessel.
Cellulose or lignocellulose itself can contain water dependent upon the source and
physical form of the cellulose.
Cellulose can, for example, contain 10-15% by weight water. Lignocellulosic materials subjected to pretreatment, for example
dilute acid pretreatment, may contain water. Typically, such incidental water is present at levels of at most about 5 weight % of all reaction components. When incidental water levels are lower than about 5 weight % of all reaction components, water may optionally be added to the initial reaction mixture up to a level of about 5 weight % with respect to all reaction components. A portion of this initially added water may be added to facilitate acid addition. Any known amounts of water in the cellulose or added to the initial reaction mixture is considered in the determination of total
water content, when water is added after the reaction is initiated. Initial
water content is preferably sufficiently low to avoid any substantial cellulose
precipitation.
Cellulose precipitation can, for example, be detected visually by cloudiness in the ionic liquid. If the
water content of the material to be hydrolyzed is over about 5% by weight, lignocellulosic materials or cellulose are optionally subjected to at least partial
drying before hydrolysis to reduce water levels to about 5 weight % or less. Water-levels in material to be hydrolyzed can be determined by any method known in the art. Water levels in the material to be hydrolyzed should be sufficiently low to avoid precipitation, when the materials are added to and at least partially dissolved in ionic liquid.
[0035]The ionic liquid
chloride salt dissolves cellulose and is believed to facilitate enhanced yield of glucose. Cellulose is introduced into the ionic liquid and vigorously stirred or mixed to aid
dissolution. Optionally, the cellulose is stirred in the ionic liquid for at least an hour prior to adding acid and initiating the reaction. Enhanced glucose yield can be obtained if the cellulose is mixed with the ionic liquid for up to 3, up to 6 or up to 9 hours prior to
initiation of reaction. This premixing of the ionic liquid with lignocellulose can be performed at ambient temperature or at a temperature above ambient up to 140 C, dependent upon the melting or
softening point of the ionic liquid. The ionic liquid should be liquid or at least softened (so that it can be mixed). More specifically, the premixing can be performed at
reaction temperature, specifically at a temperature between 70 and 140° C.
[0036]The ionic liquid
chloride salt decrystallizes lignocellulose and at least partially dissolves cellulose therein. The ionic liquid is believed to facilitate enhanced yield of glucose. Lignocellulose is introduced into the ionic liquid and vigorously stirred or mixed to aid decrystalization or
dissolution. Optionally, the lignocellulose is stirred in the ionic liquid for at least an hour prior to adding acid and initiating the reaction. Enhanced glucose yield can be obtained if the lignocellulose is mixed with the ionic liquid for up to 3, up to 6 or up to 9 hours prior to
initiation of reaction. This premixing of the ionic liquid with lignocellulose can be performed at ambient temperature or at a temperature above ambient up to 140° C., dependent upon the melting or
softening point of the ionic liquid. The ionic liquid should be liquid or at least softened (so that it can be mixed). More specifically, the premixing can be performed at
reaction temperature, specifically at a temperature between 70 and 140° C.
 Login to View More
Login to View More