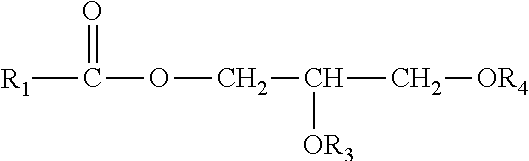
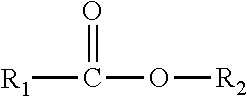
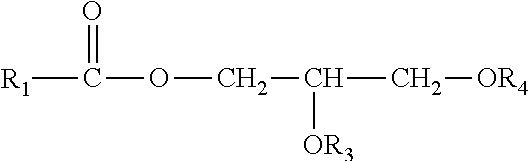
Perhydrolase providing improved specific activity
a technology of peroxycarboxylic acid and specific activity, which is applied in the direction of enzyme stabilisation, depsipeptides, and vector-based foreign material introduction, can solve the problem of reducing the amount of enzyme used to achieve the desired concentration of peroxycarboxylic acid, and achieve the effect of increasing specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Random Mutagenesis Library of Thermotoga maritima Acetyl Xylan Esterase C277S Variant
[0216]The coding sequence of a Thermotoga maritima acetyl xylan esterase (GENBANK® accession # NP—227893.1) was synthesized using codons optimized for expression in E. coli (DNA 2.0, Menlo Park, Calif.), and cloned into pUC19 between Pst1 and Xba1 to create the plasmid known as pSW202 (U.S. Patent Application Publication No. 2008-0176299). The codon-optimized sequence is provided as SEQ ID NO: 1 encoding the wild-type Thermotoga maritima acetyl xylan esterase provided as SEQ ID NO: 2.
[0217]A codon change was made in the gene using primer pairs identified as SEQ ID NO: 3 and SEQ ID NO: 4, and the QUIKCHANGE® site-directed mutagenesis kit (Stratagene, La Jolla, Calif.), according to the manufacturer's instructions, resulting in the amino acid change C2775 (SEQ ID NO: 5), to create the plasmid known as pSW202 / C277S (SEQ ID NO: 6). Plasmid pSW202 / C277S served as a template for error-pr...
example 2
Screening of Thermotoga maritima Error-prone PCR Library for Increased Enzyme Activity
[0218]Colonies were picked (automated) and placed into 96-well “master plates” containing 0.1 mL LB media supplemented with 0.1 mg ampicillin / mL and grown 16-18 h at 37° C. and 80% humidity. From each well of the master plates, 0.003 mL of culture was transferred to 96-well “induction plates” containing 0.3 mL LB media supplemented with 0.1 mg ampicillin / mL and 0.5 mM IPTG, which were incubated for 16-18 h with shaking at 37° C. and 80% humidity. Separately, 0.1 mL of 50% glycerol was added to each well of the master plates, which were stored at −80° C. as stocks. From each well of the induction plates, 0.01 mL of culture was transferred to 96-well “lysis plates” containing 0.09 mL of 56 mg / mL CELLYTIC™ Express (Sigma Aldrich, St. Louis, Mo.), which were incubated for 30 minutes at 30° C. From each well of the lysis plates, 0.01 mL of material was transferred to 96-well “assay plates” containing 0....
example 3
Production of Variant Thermotoga maritima Perhydrolases
[0219]Variant strains identified in the screen (KLP18 / pSW202 / L8R / L125Q / Q176L / V183D / F247I / C277S / P292L; KLP18 / pSW202 / A206E / C277S; KLP18 / pSW202 / S35R / H50R / C277S; KLP18 / pSW202 / K77E / A266E / C277S; KLP18 / pSW202 / F27Y / I149V / A266V / C277S / I295T / N302S; KLP18 / pSW202 / G119S / L207P / C277S; KLP18 / pSW202 / L195Q / C277S; KLP18 / pSW202 / K52E / C277S / Y298F; KLP18 / pSW202 / L125Q / T249S / C277S / A311V; KLP18 / pSW202 / F38S / V197I / N275T / C277S; KLP18 / pSW202 / K77I / K126N / C277S / K293R; and KLP18 / pSW202 / Y110F / C277S) were grown in LB media at 37° C. with shaking up to OD600nm=0.4-0.5, at which time IPTG was added to a final concentration of 1 mM, and incubation continued for 2-3 h. Cells were harvested by centrifugation and SDS-PAGE was performed to confirm expression of the perhydrolase enzyme at 20-40% of total soluble protein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| hard | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com