Method for constructing non-denatured protein hydrogel through molecular self-assembly technology
A technology of self-assembly technology and construction method, which is applied in the field of preparing non-denatured protein hydrogel materials to achieve the effect of broadening application scenarios
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
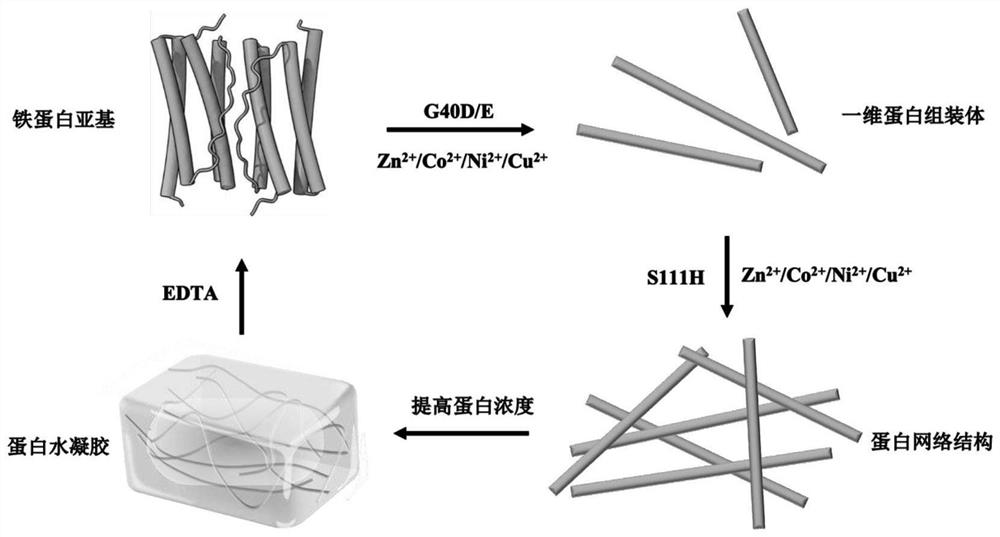
[0024] A kind of non-denatured protein hydrogel material prepared by molecular self-assembly technology, the preparation method comprises the following steps:
[0025] (a) Design and preparation of protein mutants: Using the sequence of archaeal ferritin from Thermotoga maritima (GenBank: AKE30776.1) as a template, a single point mutation was performed on the sequence by PCR gene amplification technology, and in A stop codon was added after glycine at position 150, named TmFtn△E. Then, using the sequence of TmFtn△E as a template, a single point mutation was performed on the sequence by PCR gene amplification technology to mutate the glycine at position 40 of TmFtn△E into glutamic acid / aspartic acid, which was named TmFtn△E40D / Ferritin mutants of E. The plasmid of the mutant was introduced into BL21(DE3) competent, and E. coli was amplified in LB medium containing a final concentration of 50 μg / mL Amp. When the OD600 reached about 0.8, the target was induced by adding 200 μM ...
Embodiment 1
[0044] The steps of protein expression and purification of Thermotoga maritima ferritin mutant in the present invention.
[0045] (1) Plasmid preparation and protein expression of thermotoga maritima ferritin mutant
[0046] Design and preparation of protein mutants: Using the sequence of archaeal ferritin from Thermotoga maritima as a template (GenBank: AKE30776.1), a single point mutation was performed on the sequence by PCR gene amplification technology, and the glycine at position 150 After adding a stop codon, named TmFtn△E. Then, using the sequence of TmFtn△E as a template, a single point mutation was performed on the sequence by PCR gene amplification technology to mutate the glycine at position 40 of TmFtn△E into glutamic acid / aspartic acid, which was named TmFtn△E40D / Ferritin mutants of E. The mutant plasmid was introduced into BL21(DE3) competent cells. The specific operation was to add 5 μL of the mutant plasmid to 100 μL of competent cells. After standing on ice...
Embodiment 2
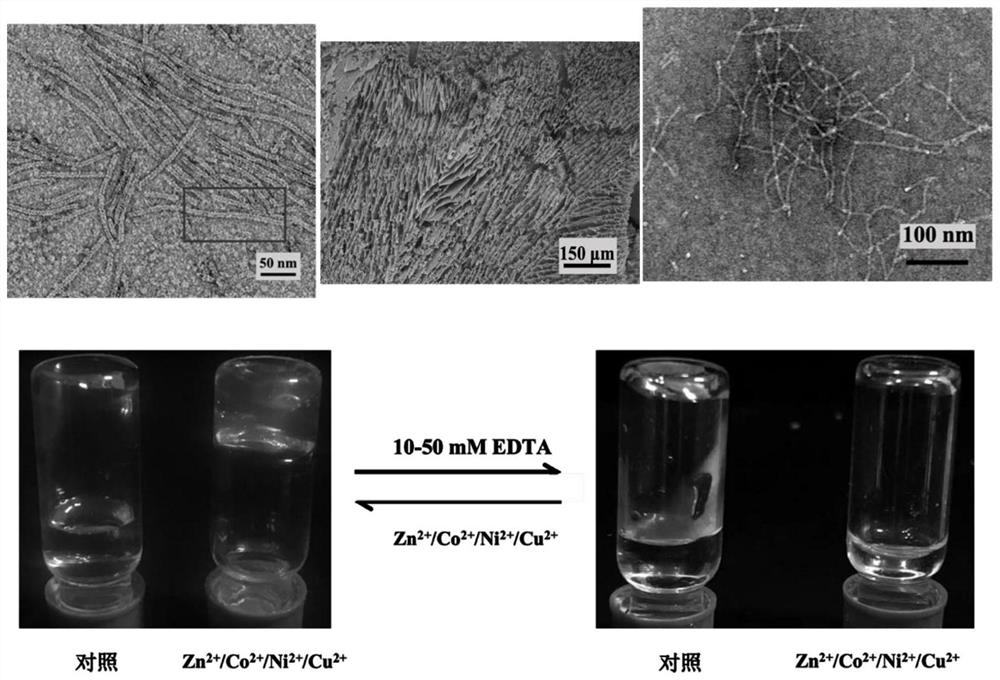
[0050] In the present invention, the construction of the thermotoga maritima one-dimensional protein assembly, the formation of the protein network structure and the preparation of the protein hydrocoagulant are carried out in various steps.
[0051] (1) Construction of one-dimensional and network structural protein assemblies of Thermotoga maritima
[0052] Thermotoga maritima ferritin TmFtn△E 40D / E After separation and purification, dialyze into a buffer containing 10-50mM EDTA (pH8.0, 25mM HEPES, 250mM NaCl) to completely remove free metal ions. The protein solution was then dialyzed again into buffer (pH 8.0, 25 mM HEPES, 250 mM NaCl) to remove EDTA and its formed metal complexes. The protein concentration was measured by the Lowery method, and a protein stock solution with a final concentration of 1 mg / mL was prepared. Use acidified water to prepare a zinc / cobalt / nickel / copper ion solution with a mother liquor concentration of 100mM, dilute the corresponding multiples a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com