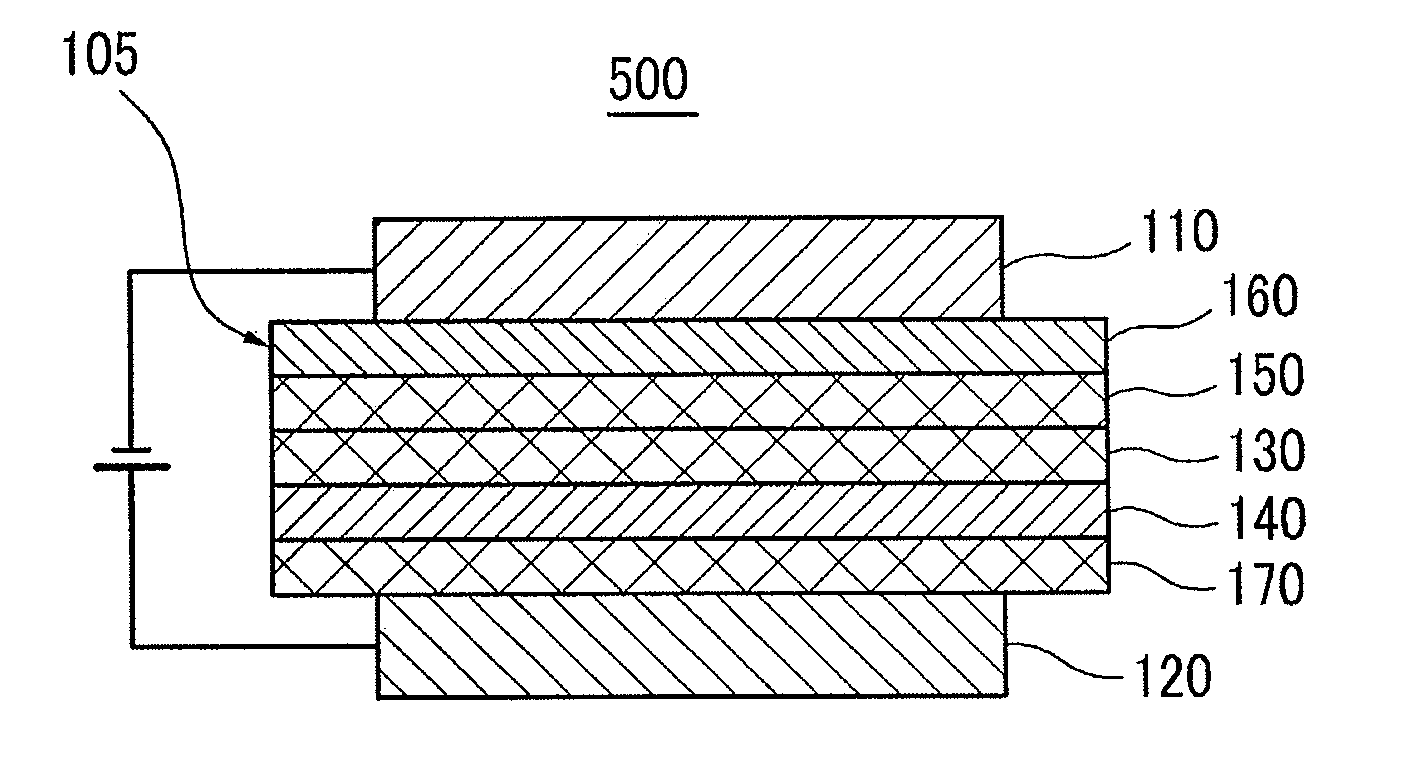
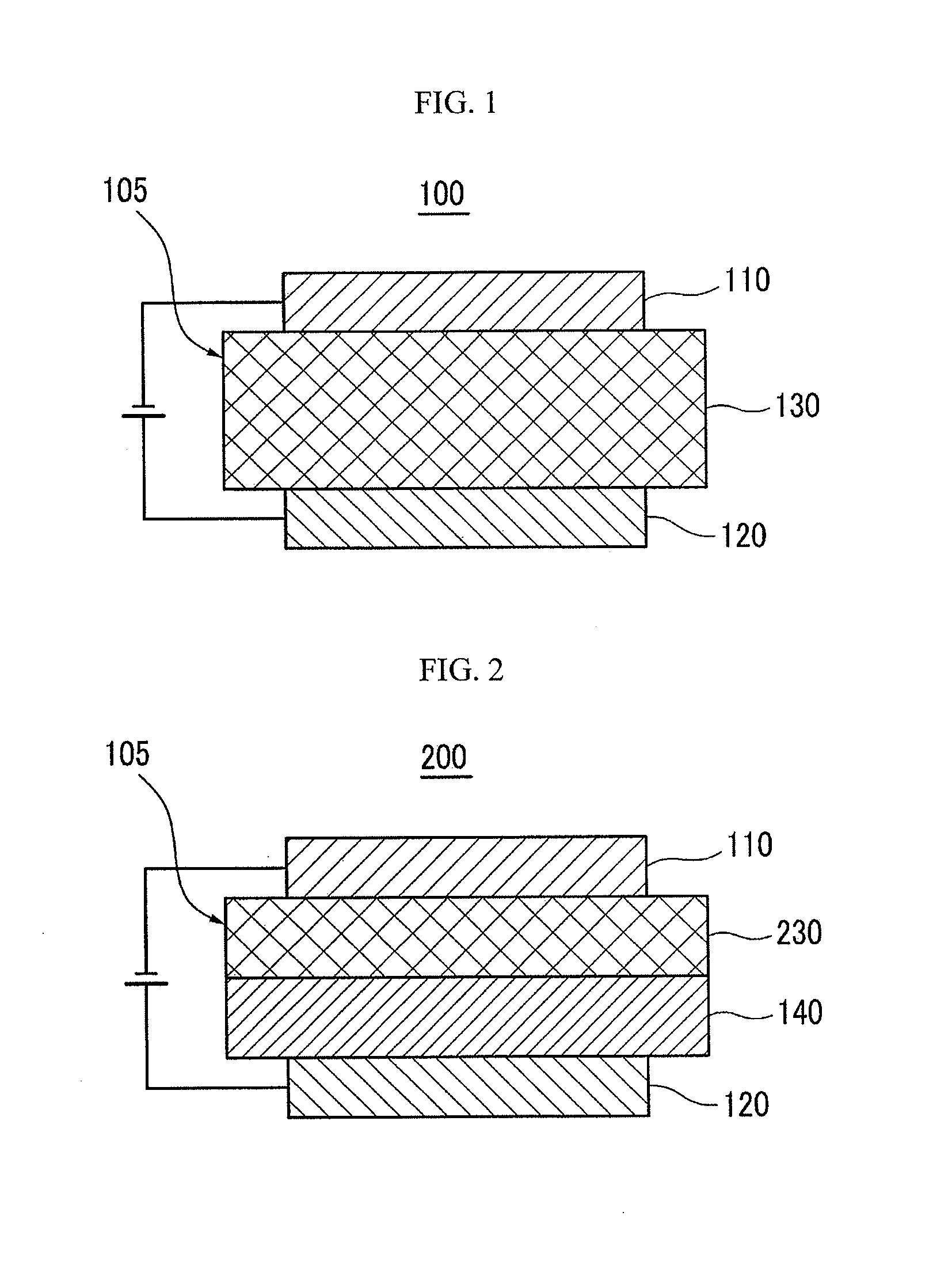
Material for organic photoelectric device, and organic photoelectric device thereby
a photoelectric device and organic technology, applied in triarylamine dyes, anthracene dyes, other domestic articles, etc., can solve the problems of limit in luminous efficiency, and achieve the effects of high efficiency, good hole and electron transporting properties, and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Material for Organic Photoelectric Device
example 1-1
Synthesis of Compound (16)
[0152]The following compound (16) was synthesized as shown in the following Reaction Scheme 1.
[0153]Step 1: Synthesis of Intermediate Product (A)
[0154]40.4 g (241 mmol) of carbazole, 38.0 g (121 mmol) of 1,3,5-tribromobenzene, 2.99 g (30 mmol) of cuprous chloride, and 66.7 g (483 mmol) of potassium carbonate were suspended in 171 ml of dimethylsulfoxide, and refluxed under a nitrogen atmosphere for 8 hours while heating.
[0155]The refluxed reaction solution was cooled to room temperature and recrystallized with methanol. The precipitated crystals were separated by filtration and the obtained residue was purified by silica gel column chromatography, providing 36.7 g of the first crystalline intermediate (A) (yield 62.4%).
[0156]Step 2: Synthesis of Intermediate Product (B)
[0157]35.0 g (72 mmol) of intermediate product (A) was dissolved in 350 ml of tetrahydrofuran, and then 61.5 ml (98 mmol) of n-butyl lithium hexane solution (1.6 M) was added thereto under an...
example 1-2
Synthesis of Compound (24)
[0170]The following compound (24) was synthesized as shown in the following Reaction Scheme 2.
[0171]Step 1: Synthesis of Intermediate Product (F)
[0172]15 g (63 mmol) of 1,3-dibromobenzene, 7.66 g (44 mmol) of 1-naphthalene boronic acid, 17.58 g (127 mmol) of potassium carbonate, and 1.83 g (50 mmol) of tetrakis(triphenylphosphine)palladium were suspended in 200 ml of tetrahydrofuran, 200 ml of toluene, and 50 ml of purified water, and refluxed under a nitrogen atmosphere for 24 hours while heating.
[0173]The refluxed reaction solution was cooled to room temperature, and the reaction solution was separated into two layers. The solution obtained after the organic solvent of the organic layer was removed under a reduced pressure was separated using column chromatography (hexane), and then solvent was removed to obtain 15 g (83%) of a gel-type intermediate product (F).
[0174]Step 2: Synthesis of Intermediate Product (G)
[0175]7 g (24 mmol) of the intermediate prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal quantum efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com