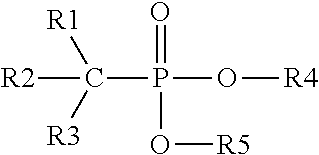
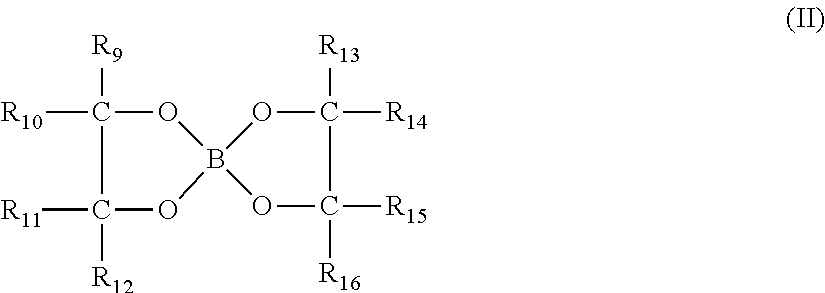
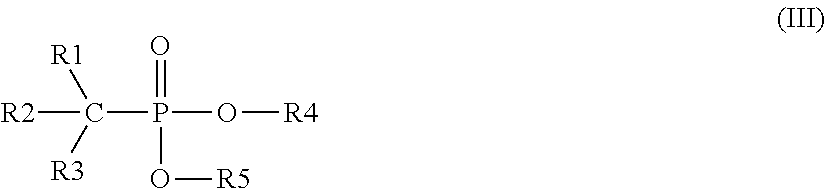Method of removing inorganic scales
a technology of inorganic scales and inorganic sand, which is applied in the direction of fluid removal, borehole/well accessories, chemistry apparatus and processes, etc., can solve the problems of limiting the productive (or injective) capacity of the well, loss of formation permeability, and formation damage, so as to reduce the amount of clay in the well, inhibit or prevent swelling, and control the effect of clay migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-6
[0062]Analytical grade carbonate powder was exposed to an aqueous hydrofluoric acid solution at 70° F. The un-dissolved solid or precipitate was analyzed by X-ray diffraction technique (XRD). Table 1 presents the results of these tests wherein pH A represents the pH at the beginning of the testing and pH B represents the pH at the end of the testing.
TABLE 1Ex.CaCO3No.CompositionpH ApH BaddedCommentsComp.HF acid2.22.20.4 g / 100 ccAll carbonateEx. 1dissolved andCaF2 precipitateformed within 5minutes.Comp.HF acid1.91.90.4 g / 100 ccAll carbonateEx. 23% Dequestdissolved and2010CaF2 precipitateformed within 5minutes.Comp.HF acid2.2>4.00.4 g / 100 ccAll carbonateEx. 32.8 g / 100 ccdissolved andBoric acidCaF2 precipitateformed within 5minutes.4HF acid1.61.60.4 g / 100 ccAll carbonate3% Dequestdissolved and2010no precipitate2.8 g / 100 ccformed over 4Boric acidhours.5HF acid1.61.61.0 g / 100 ccAll carbonate3% Dequestdissolved and2060Sno precipitate4.2 g / 100 ccformed over 24Boric acidhours.6HF acid1.61.6...
example 7
[0063]The dissolution effect of the compositions of Examples 1-6 was illustrated on a formation containing calcareous minerals as follows. A composition consisting of 75 wt. % quartz, 5 wt. % kaolinite, 10 wt. % potassium-feldspar and 10 wt. % calcium carbonate (powder) was prepared. The composition was tested for its solubility in a HF acid at 150° F. over 4 and 24 hrs. After solubility testing, the un-dissolved solid or precipitate was analyzed. The experimental conditions and results are set forth in Tables 2-5. Table 2 represents the 4 hour solubility testing of the formation composition at 150° F. Tables 3-5 represent the 4 and 24 hour solubility testing of the formation composition at 150° F.
TABLE 2HF acidHF acid3% Dequest3% DequestHF acidHF acid2010HF acid2060S3% Dequest2.8 g / 100 cc2.8 g / 100 cc3% Dequest2.8 g / 100 ccAcid2010Boric acidBoric acid2060SBoric acidpH1.9 / 1.92.2 / 5.51.6 / 1.91.6 / 1.61.0 / 1.3before / afterSolubility, %14.94.414.414.79.6Quartz8779918988Plagioclasend11nd1K-feld...
example 8
[0065]A coreflood study was conducted using a Bandera Sandstone core at 180° F. The composition of Bandera Sandstone is set forth in Table 6:
TABLE 6Mineral CompositionWt %Quartz61Feldspar15Dolomite5Illite12Kaolinite4Chlorite2
Table 7 shows the composition of acids systems used in the coreflood experiment wherein HV represents an organophosphonate.
TABLE 7Pre-Flush acidMain acidPost-Flush acidSystemsystemsystemHF, wt %—3—HCl, wt %10210HV, gpt—60—Iron control agent, gpt—10—Corrosion inhibitor, gpt 22 2Acetic acid, wt %—3—Boric Acid, ppt—60—
Five fluid stages were injected in the following sequence:[0066]1. 5 wt % NH4Cl solution to measure initial core permeability.[0067]2. 4 pore volumes (PV) of Pre-Flush acid system.[0068]3. 4 PV of Main acid system.[0069]4. 4 PV of Post-Flush acid system.[0070]5. 5 wt % NH4Cl solution to measure initial core permeability.
The results of two coreflood experiments are shown in Table 8. All acid solutions injected in the second experiment contained the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com