Controlled release doxycycline
a technology of doxycycline and doxycycline, which is applied in the direction of biocide, plant growth regulator, animal husbandry, etc., can solve the problems of reducing the availability of free fatty acids involved in inflammation, gastrointestinal irritation and nausea undesirable, and the class of compounds suffers from a major drawback associated with administration, so as to increase the efficacy of the antibiotic and reduce the release level. , the effect of increasing the tolerability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Controlled Release Doxycycline Hyclate Pellets Core Preparation
[0169]The core is formed in a wet granulation process using a saturated solution of sodium chloride and in a high shear mixer.
[0170]The mixture is then extruded using a screen size of between 0.4 and 1.5 mm. The extrudate is then marumerised to produce rounded core elements. The core elements are dried in a fluidized bed or an oven.
[0171]Stabilizing Coat Application
[0172]A stabilizing coat is applied to the core using a fluidized bed coating process.
[0173]The stabilizing coat contains hydroxypropylmethyl cellulose and talc in a 2:1 mixture.
[0174]The desired polymer coat weight (i.e. the weight of the polymer only, not including the talc) is between 3% and 5% of the total weight of the core element and the stabilizing coat.
[0175]The polymer coat weight will vary due to a number of factors, such as the efficiency of the coating process, the batch of raw materials, etc.
[0176]Modified Release Coat Application
[...
example 2
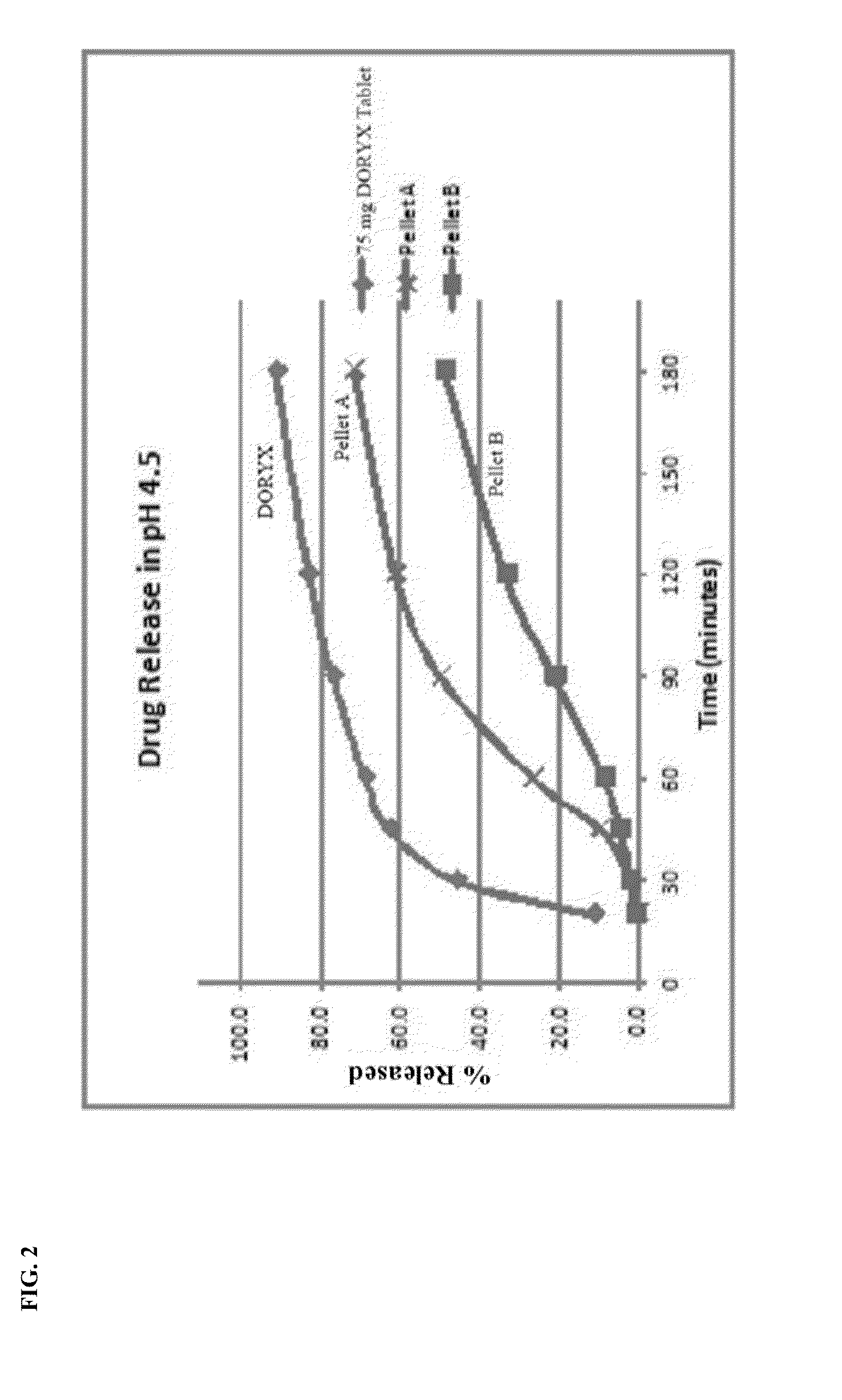
Dissolution Profiles of Control (DORYX), Pellet A Formulation, & Pellet B Formulation Acidic Dissolution Profile
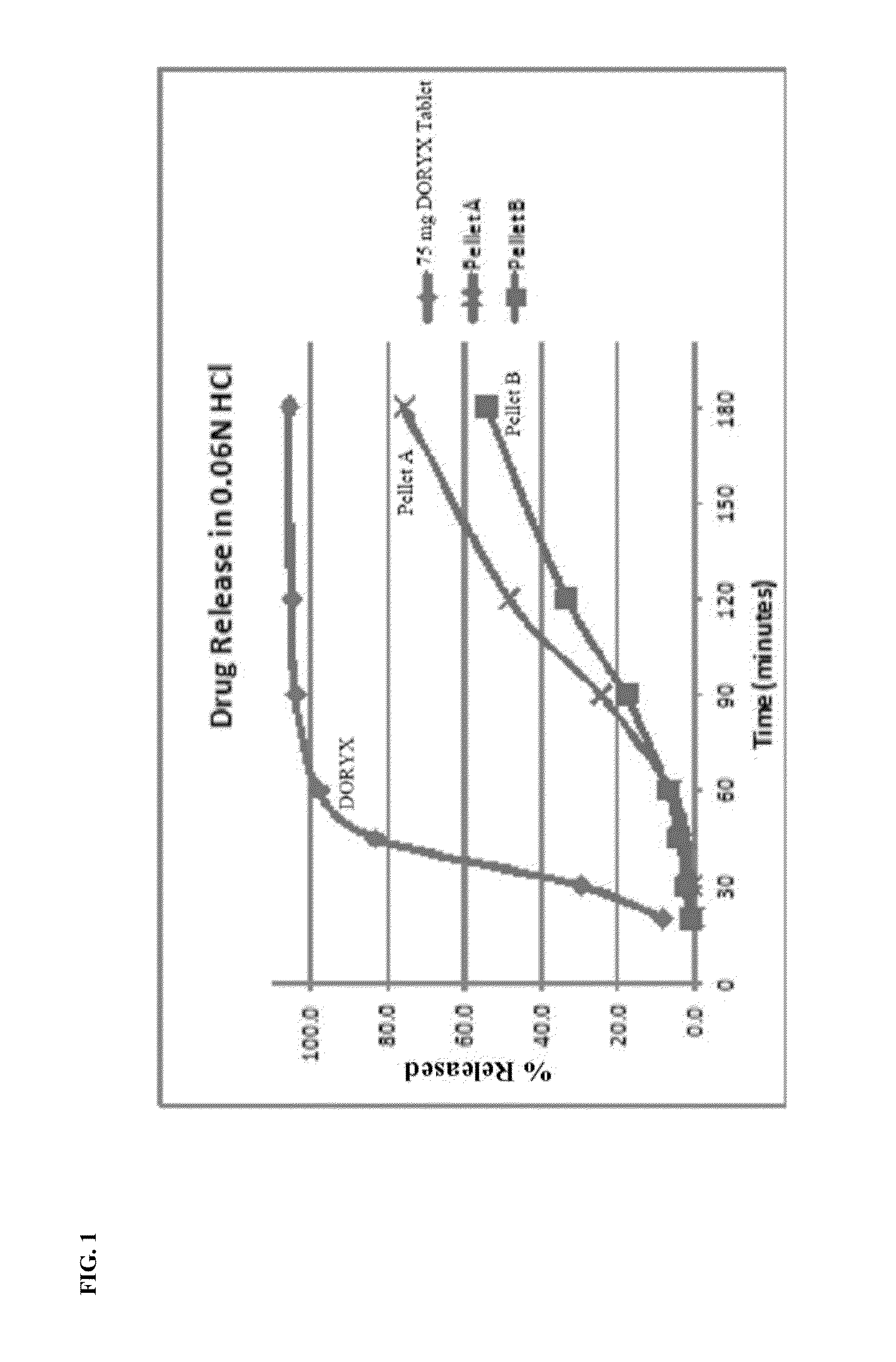
[0183]The acid environment dissolution profile of pellet A and B's formulation can be seen in FIG. 1.
[0184]As can be seen in FIG. 1, the formulations of pellet A and B release less than 10% of the doxycycline hyclate in an acid environment (pH of approximately 1.2) after 60 minutes of exposure under standard USP conditions.
[0185]The slow rate of release of doxycycline, in a pH of approximately 1.2, exhibited by the pellet formulations according to the disclosure is in stark contrast to the dissolution profile of the commercially available DORYX.
[0186]As can be seen in FIG. 1, the DORYX tablet released approximately 98%-100% of the doxycycline hyclate at 60 minutes.
[0187]Thus, the controlled release doxycycline formulations of the disclosure exhibit a beneficial and superior dissolution profile in acidic conditions, as compared to a commercially available doxycycline formul...
example 3
Clinical Data
[0195]The aforementioned controlled release pellet formulations (A and B) were loaded into capsules and administered to 10 test subjects to obtain in vivo data.
[0196]It is also contemplated that pellet formulations A and B may be formulated into tablets.
[0197]After all 10 completed subjects were analyzed; the following Geometric Mean Ratio (GMR) and Intra-Subject Coefficient of Variation (CV) were obtained for the two Test Formulations.
TABLE 2In Vitro Pharmacokinetics DataFormulationParameterGMR (%)CV (%)Pellet AAUC0-∞89.6417.24AUC0-t89.5817.71Cmax88.0819.87Pellet BAUC0-∞106.9017.24AUC0-t106.3717.71Cmax103.7319.87
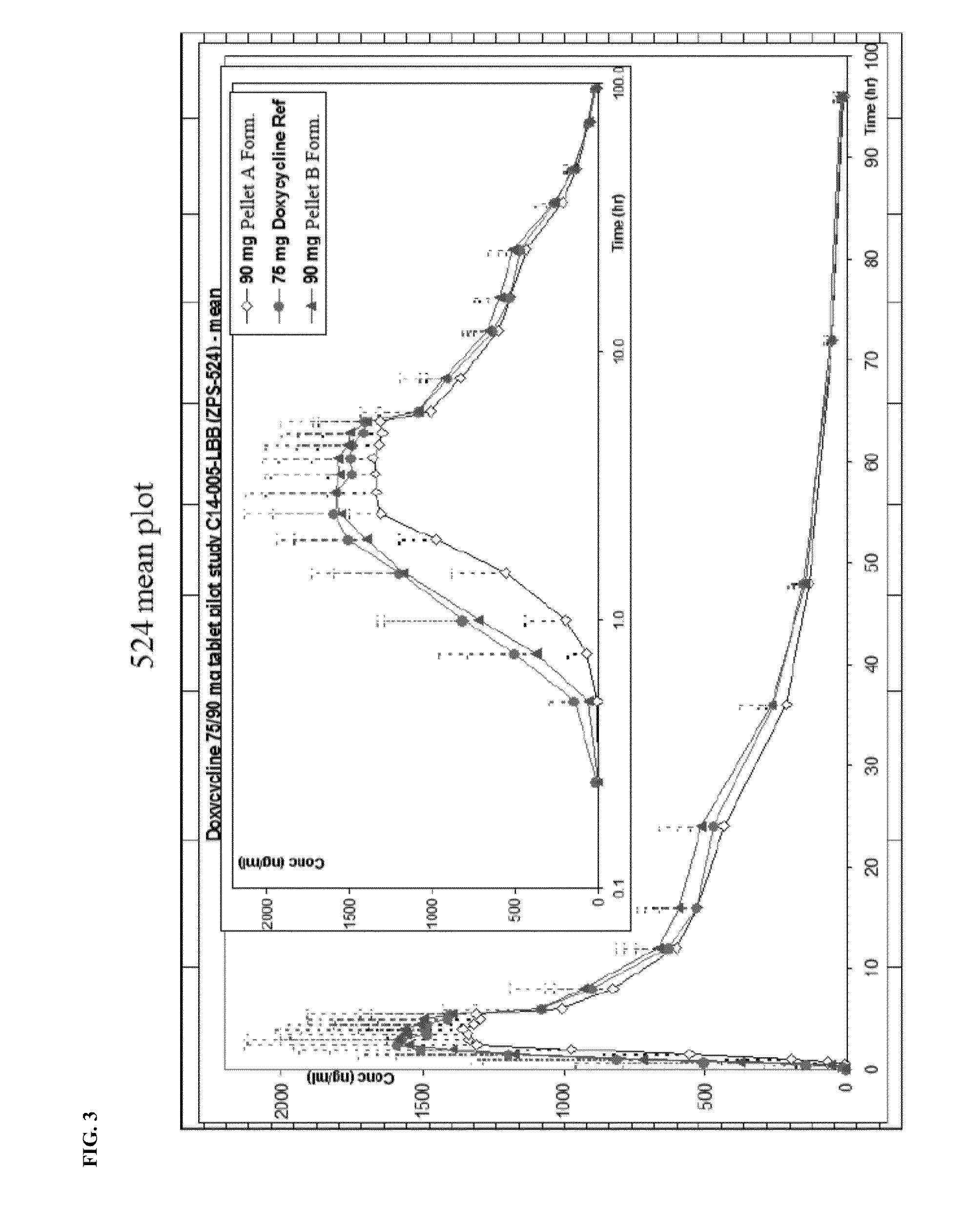
[0198]Further, FIG. 3 and FIG. 4 illustrate the concentration of active substance in ng / mL of the two tested pellet A and pellet B formulations as compared to a doxycycline (DORYX) control.
[0199]As shown in FIG. 3, 90 mg controlled release pellet formulations (A and B) of the present invention have average AUC0-t values (ng·hr / ml) which differ from that of conv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com