Hydrometallurgical process for nickel oxide ore
a technology of nickel oxide ore and hydrometallurgical process, which is applied in the direction of process efficiency improvement, etc., can solve the problems of increasing the number of equipment to increase cost and labor, increasing the cost of raw materials for metal smelting, and increasing so as to reduce the amount of final neutralized residue, reduce the amount of leach residue, and reduce the effect of facilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
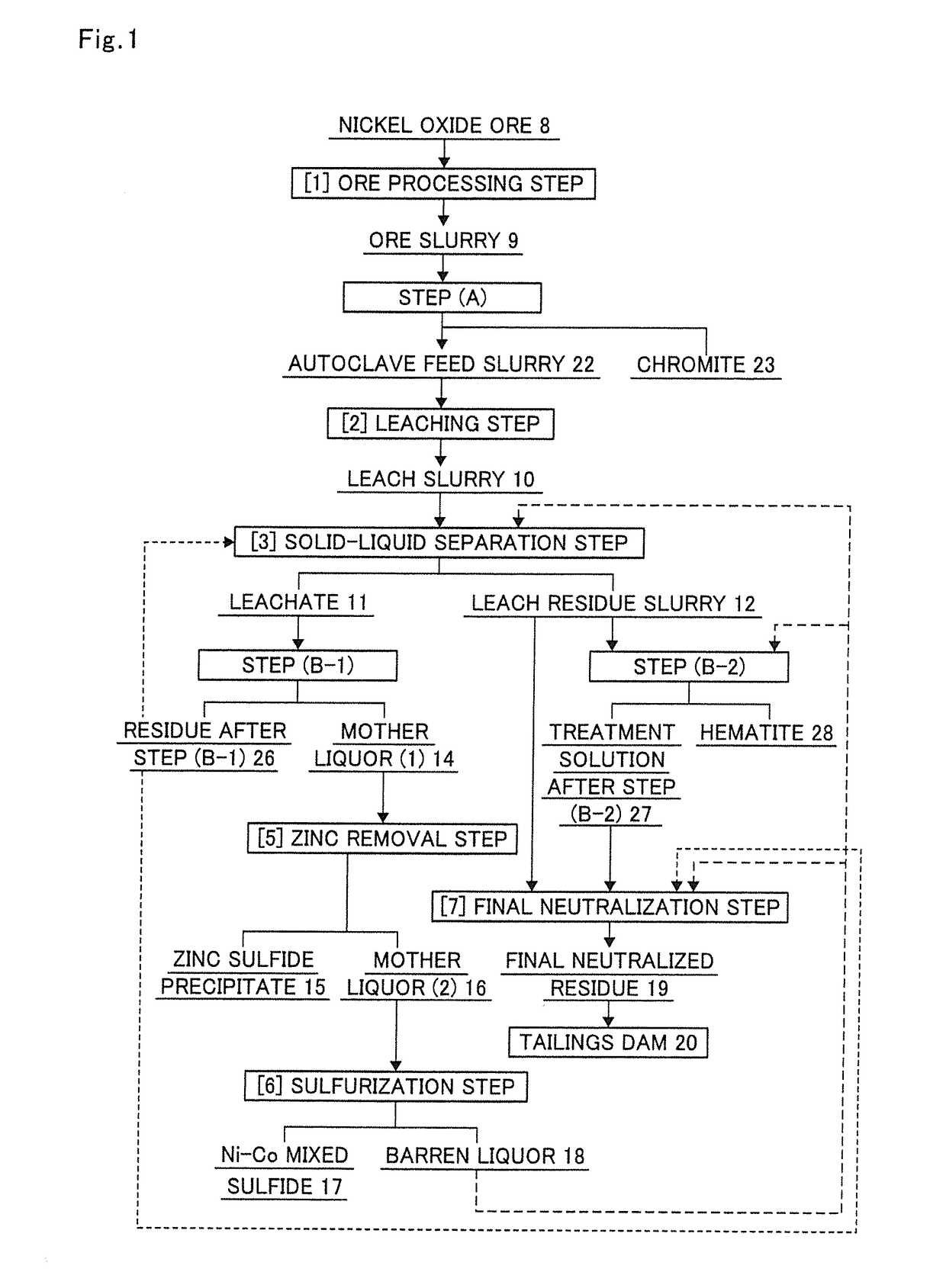
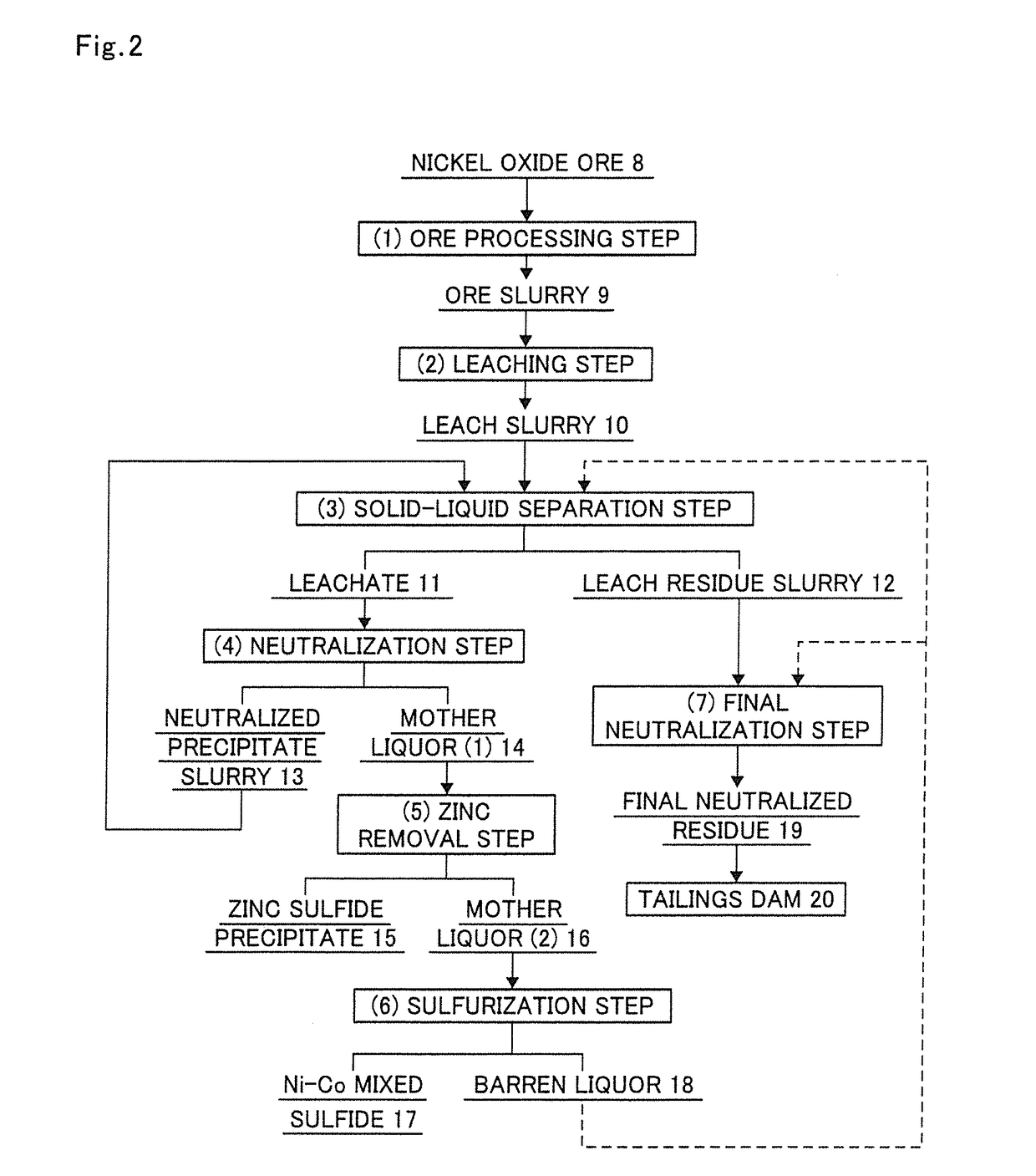
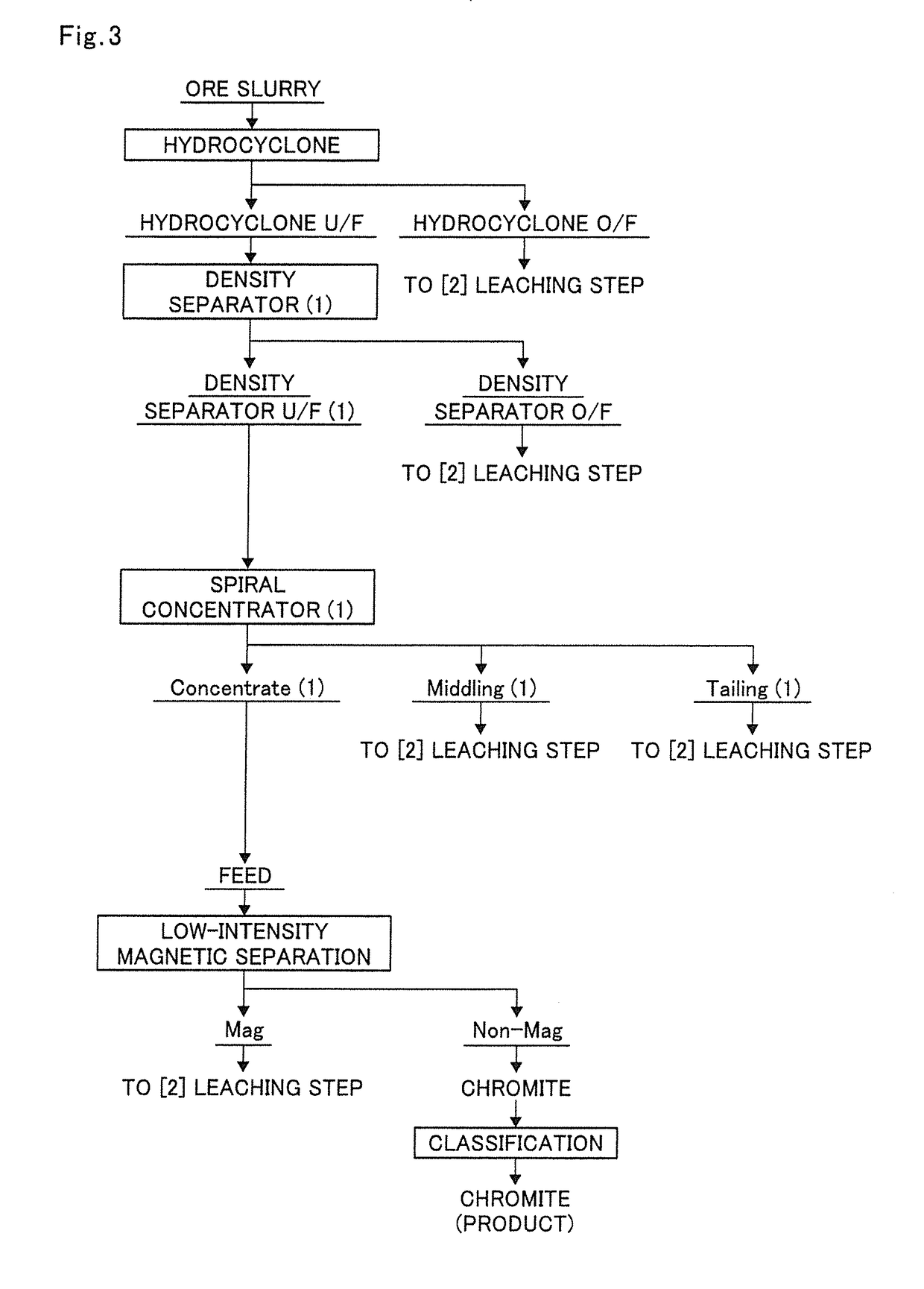
[0189]In the production flow of the present invention in FIG. 1, the ore slurry 9 was subjected to step (A) according to the execution flow chart shown in FIG. 3, in which the ore slurry 9 was subjected to classification with a hydrocyclone to separate goethite and then subjected to specific gravity separation once with a density separator and a spiral concentrator in combination in this order.
[0190]The ore slurry having the composition shown in Table 3 was classified using a hydrocyclone (manufactured by Daiki Ataka Engineering Co., Ltd., Model MD-9) as a classifier used in step (A).
[0191]In Example 1, the classification was performed under the conditions of a slurry concentration of 15% by weight, a slurry temperature of normal temperature, and an operating pressure of 0.2 MPa.
[0192]The ore slurry composition and the composition of the underflow from the hydrocyclone (hydrocyclone U / F) are shown together in Table 3. Note that the unit in the following tables is % by weight.
TABLE 3...
example 2
[0224]In the production flow of the present invention in FIG. 1, the ore slurry was subjected to step (A) as shown in the execution flow chart of step (A) in FIG. 4, in which the ore slurry was subjected to specific gravity separation repeatedly twice with a density separator and then subjected to specific gravity separation with a spiral concentrator.
[0225]First, the ore slurry having the composition shown in Table 9 was classified using a hydrocyclone (manufactured by Daiki Ataka Engineering Co., Ltd., Model MD-9) as a classifier used in step (A).
[0226]In Example 2, the classification was performed under the conditions of a slurry concentration of 15% by weight, a slurry temperature of normal temperature, and an operating pressure of 0.2 MPa.
[0227]The ore slurry composition and the composition of the hydrocyclone U / F are shown together in Table 9. Note that the unit in the following tables is % by weight.
TABLE 9Cr2O3SiO2FeNiOre slurry2.54.451.51.2Hydrocyclone U / F13.56.045.20.8Unit...
example 3
[0270]The overflow of the hydrocyclone and the overflow of the density separator in Example 1 were charged into an autoclave at a solid weight ratio of 77:15, and thereto was added 98% sulfuric acid. The resulting mixture was subjected to high pressure acid leach under the following conditions to produce a leach slurry 10.
[0271]Further, the produced leach slurry was separated into a leachate 11 and a leach residue slurry 12 by a solid-liquid separation step.
[Leaching Conditions]
[0272]Leaching temperature: 245° C.
[0273]Leaching time: 60 minutes
[0274]Final (at the completion of leaching) free sulfuric-acid concentration: 40 [g / L]
[0275]Slurry concentration: 30% by weight
[0276]Autoclave volume: 5 L
[0277]Next, in order to know the Cr2O3 grade in the leach residue slurry 12, Mg(OH)2 slurry having a concentration of 20% by weight as a neutralizing agent was added to the leach residue slurry 12 to neutralize the leach residue slurry so that it might have a pH of 2.5 at 70° C.
[0278]Next, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com