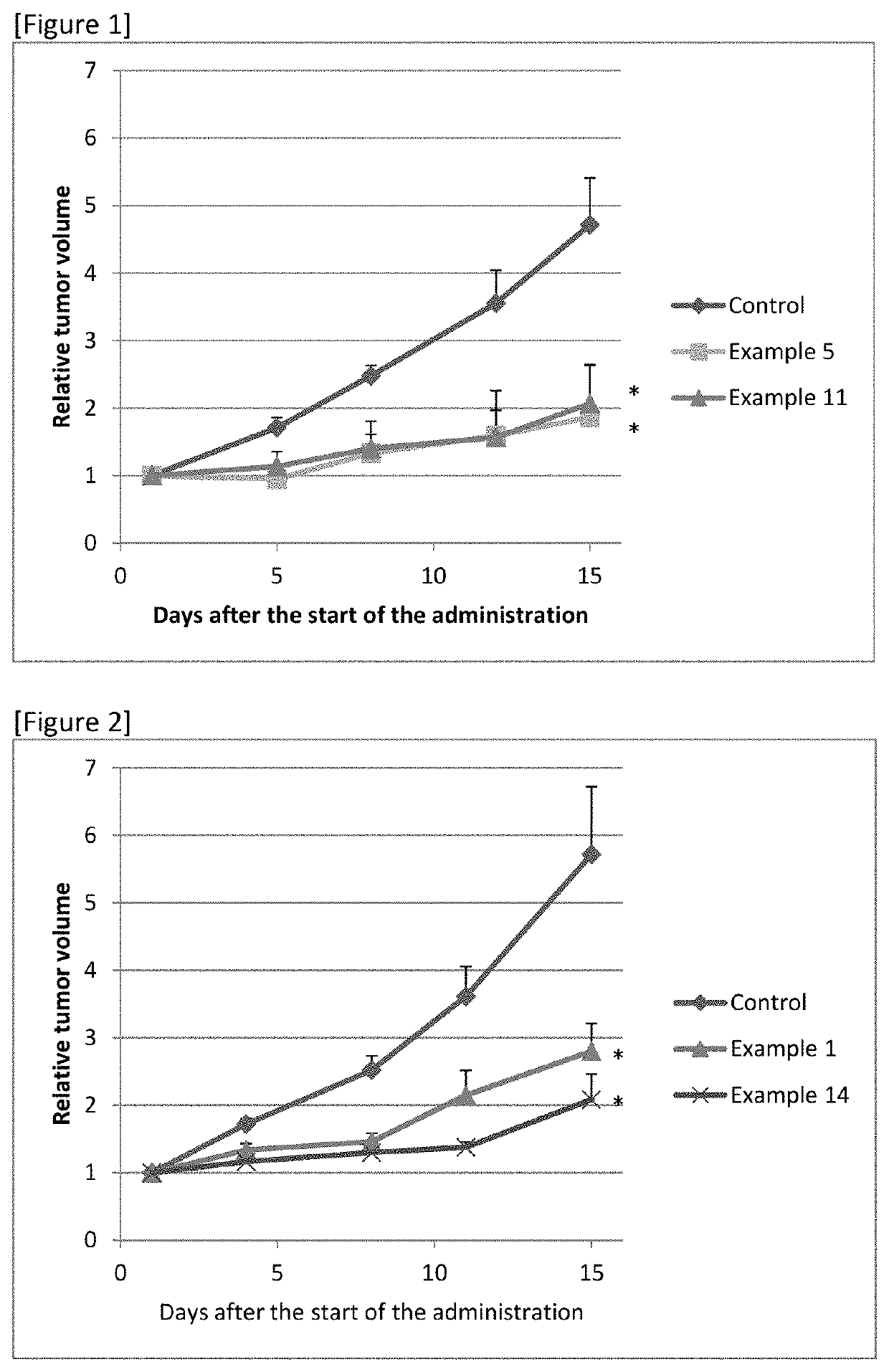
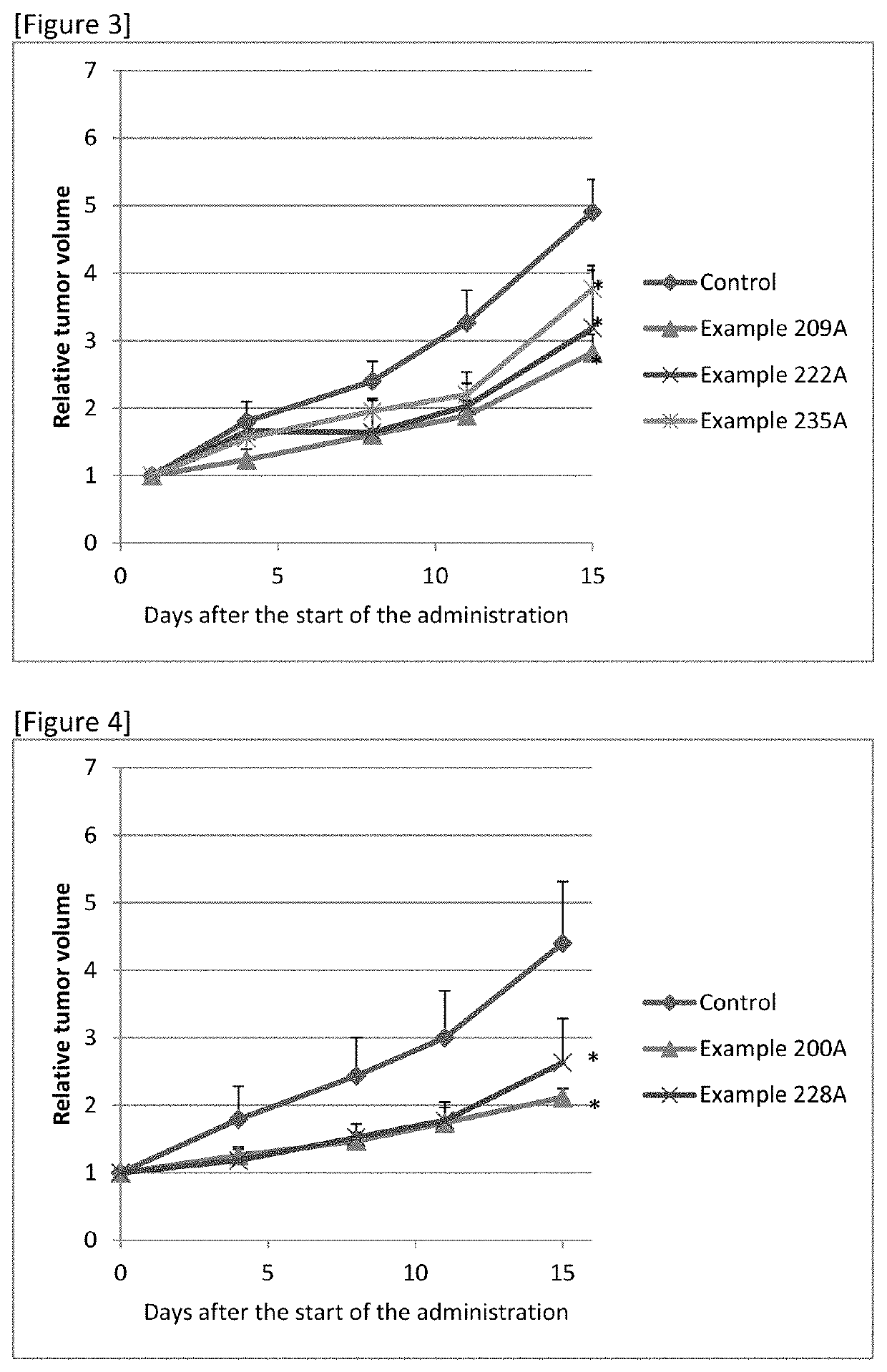
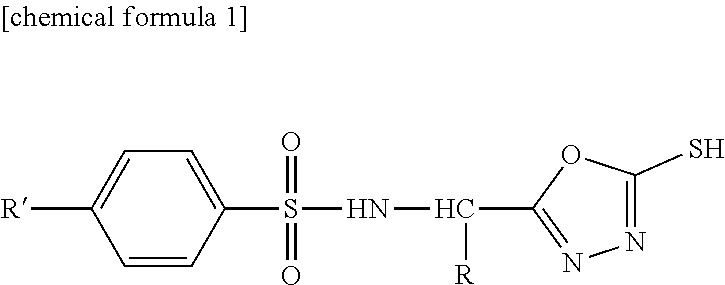
Sulfonamide Compound or Salt Thereof
a technology of sulfonamide and compound, applied in the field of new sulfonamide compound, can solve the problems of poor rnr inhibitory activity, limited effect, and difficult tolerance to hu use, and achieve excellent rnr inhibitory activity and growth inhibitory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0477]The present invention is described below in more detail with examples and test examples, but the present invention is not intended to be limited to these examples.
[0478]Various reagents used in the examples were commercially available products, unless otherwise stated. Biotage Ltd. SNAP-ULTRA (registered trademark) Silica prepacked column was used for a silica gel column chromatography, or Biotage made SNAP KP-C18-HS (registered trademark) Silica prepacked column was used for a reverse phase silica gel column chromatography. HPLC purified by preparative reverse phase column chromatography was performed under the following conditions. Injection volume and gradient was carried out appropriately.
[0479]Column: YMC-Actus Triart C18, 30×50 mm, 5 μm
[0480]UV detection: 254 nm
[0481]Column flow rate: 40 mL / min
[0482]Mobile phase: water / acetonitrile (0.1% formic acid)
[0483]Injection amount: 1.0 mL
[0484]Gradient: water / acetonitrile (10% to 90%)
[0485]AL400 (400 MHz; JEOL (JEOL)) and Mercury...
reference example a1 2
-(1-bromoethyl)-1-fluoro-3,4-dimethylbenzene
[0493]
Step 1
1-(6-fluoro-2,3-dimethylphenyl)ethanol
[0494]After dropping a diethyl ether solution of methylmagnesium bromide (3.0 M, 70 mL) to a THF solution of 6-fluoro-2,3-dimethyl-benzaldehyde (22.0 g) (300 mL) at 0° C., the reaction mixture was stirred at room temperature for 1 hour. Under ice-bath condition, a saturated aqueous ammonium chloride solution (150 mL) was added dropwise, and ethyl acetate (200 mL) was added, and the resultant was separated into different layers. The organic layer was successively washed with HCl (1M, 200 mL), water (200 mL) and saline (200 mL), and then dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 1-(6-fluoro-2,3 dimethylphenyl)ethanol (23.7 g).
(Step 2)
[0495]Phosphorus tribromide (26.5 mL) was added dropwise at 0° C. to a chloroform solution (120 mL) of 1-(6-fluoro-2,3-dimethylphenyl)ethanol (23.7 g) obtained in the above Step 1, and the reaction solution was stir...
reference examples b2
to B6
[0501]According to the methods of Reference Example B1 Steps 1 and 2 and Reference Example A1 Steps 1 and 2, the following compounds of Reference Examples B2 to B5 were synthesized. Also, according to the methods of Reference Example B1 Step 1, and Reference Example A1 Steps 1 and 2, the compound of Reference Example B6 was synthesized.
TABLE 2Reference ExampleStarting MaterialSynthesized CompoundB2B3B4B5B6
Reference Example C1 7-(1-chloroethyl)-1-methyl-2,3-dihydro-1H-indene
[0502]
[0503]Sodium borohydride (261 mg) was added to a methanol solution (5.0 mL) of 1-(3-methyl-2,3-dihydro-1H-inden-4-yl)ethanone (1.0 g), and the mixture was stirred at room temperature for 30 minutes. The reaction solution was added to water (10 mL) and then extracted twice with ethyl acetate (20 mL). The combined organic layer was washed with saturated saline (20 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was dissolved in dichloromethane (5.0 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com