Polymerizable biodegradable polymers including carbonate or dioxanone linkages
a biodegradable, carbonate or dioxanone technology, applied in the direction of biocide, prosthesis, bandages, etc., can solve the problems of inherently difficult sealing or plugging holes in lung tissue, polymerizable groups are revealed on the polymer, etc., to achieve the effect of increasing the viscosity of the macromer, reducing the degradation time of the polymer, and increasing the elasticity of the polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0120] General Synthesis of Macromers: Melt Method.
[0121]Methods analogous to those described in U.S. Pat. No. 4,526,938 to Churchill et al. were used to form derivatized PEG by the melt method. Polyethylene glycol (PEG) was obtained commercially. The molecular weight listed on the label was assumed to be the molecular weight of the material. The PEG was optionally dissolved in methanol and purified by passage over an ion exchange resin, and dried.
[0122]Purified or as-supplied PEG was charged to a reactor, optionally with a small amount of xylene, and heated for five to six hours at about 110° C. (note: all temperatures herein are in degrees Celsius) under vacuum to complete removal of water. After cooling under vacuum, the flask was placed in a glove bag, the materials for forming the biodegradable linkages (including T (trimethylene carbonate) and L (lactide)) were added to the PEG, and the temperature was raised to about 160-165° C. under an argon blanket.
[0123]After dissolution ...
example 2
[0126] Synthesis of PEG-TMC and PEG-TMC-Lactide Macromers.
[0127]35K(T8)A2 (“35KT” in the examples below) was made from purified PEG of nominal molecular weight 35,000 by the melt procedure as described above. T was charged into the reactor at a nominal molar ratio of 13:1 to the PEG to obtain this result. The final actual acrylate incorporation averaged 1.6 per PEG molecule, or about 2 acrylates per macromer.
[0128]35K(T7L2)A2 (“35KTL”) contained about 7 T units (6.88 measured) and 2 lactate units (1.86 measured) as synthesized. (Note that there are 2 lactate units per lactide molecule.) T and L were charged at nominal molar ratios of 10:1 and 3:1 relative to PEG.
[0129]20K(T30L15)A2 contained about 30 T units and 15 lactide units per 20,000 D PEG molecule. The actual acrylate to PEG ratio was 1.42.
example 3
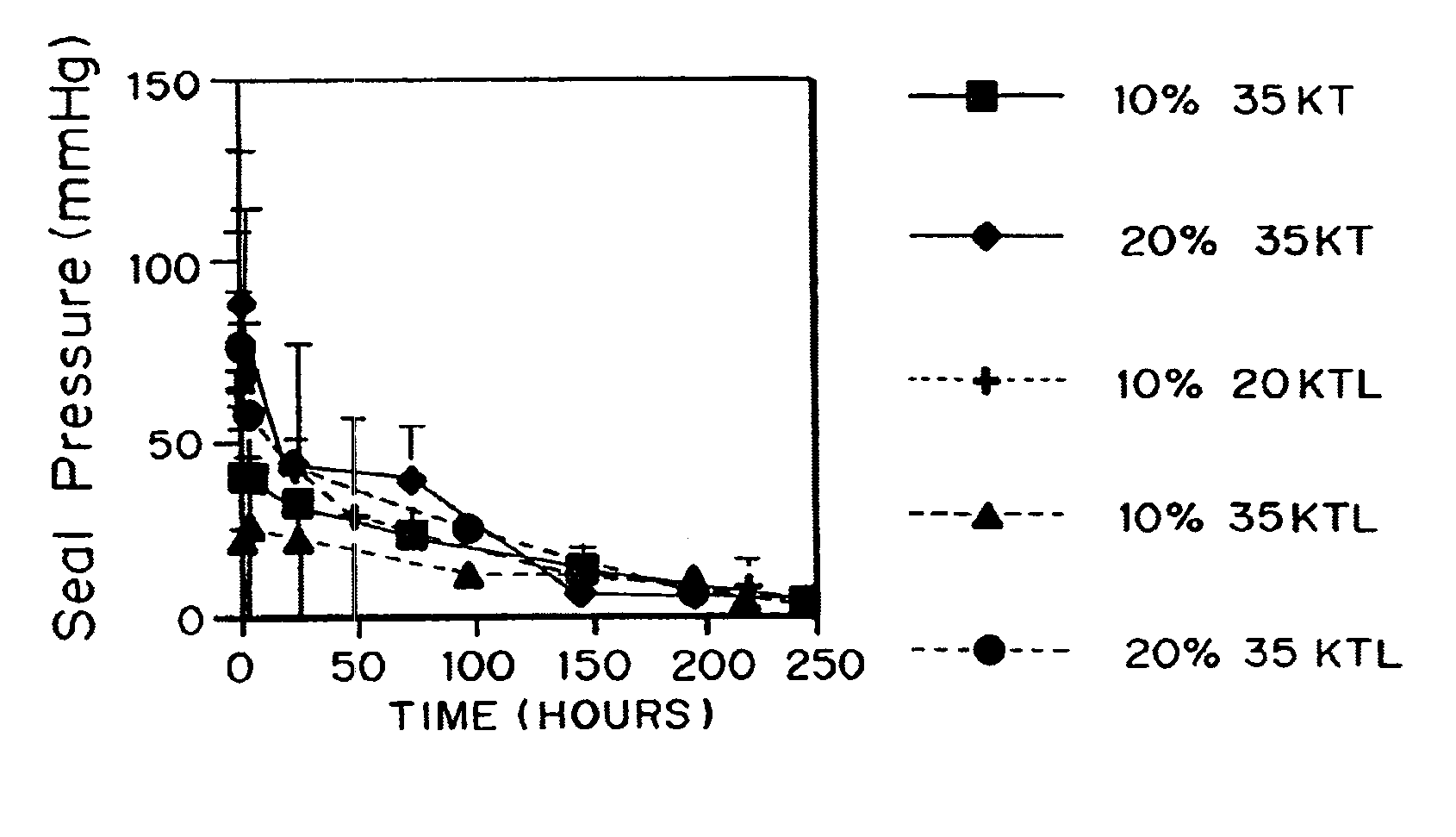
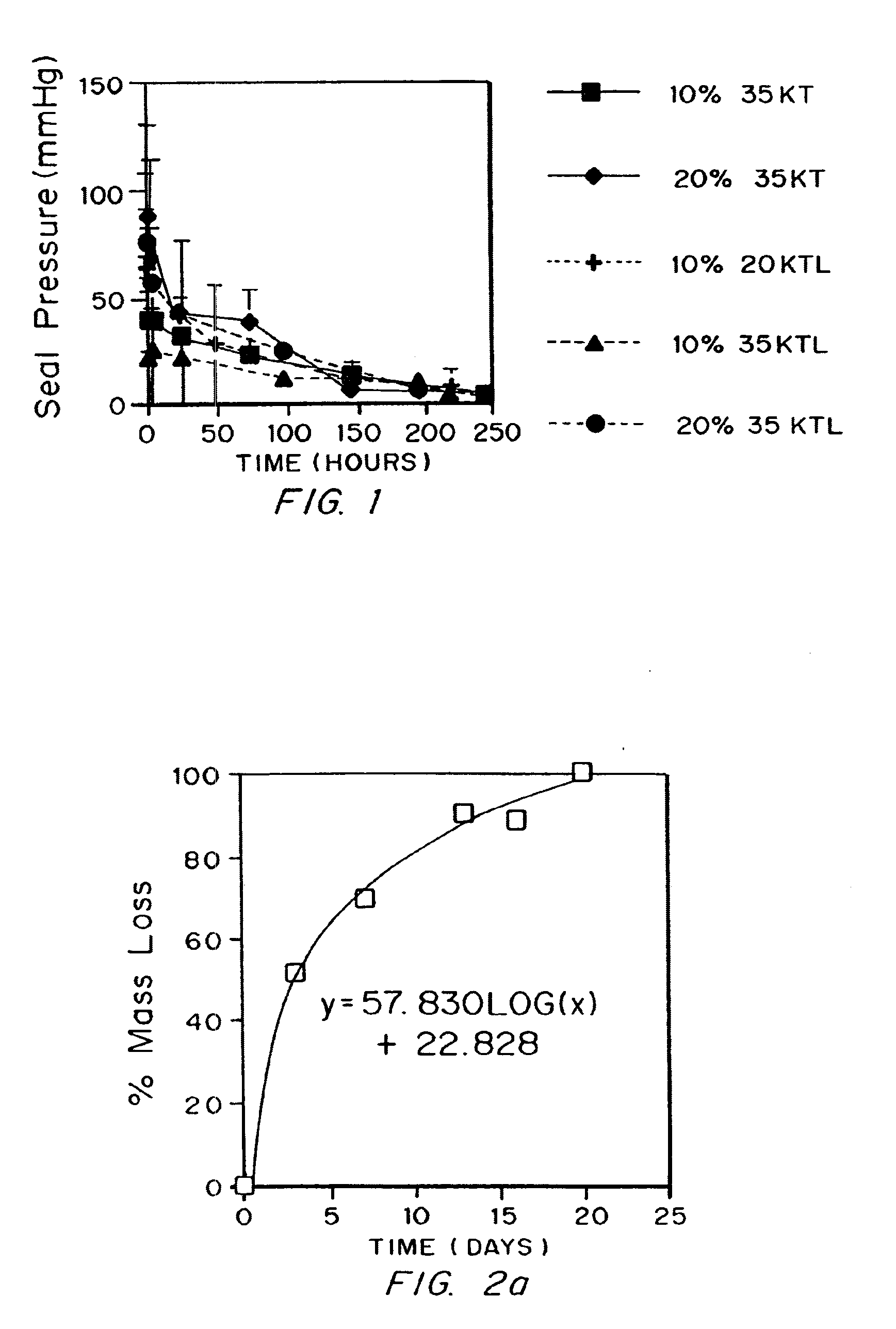
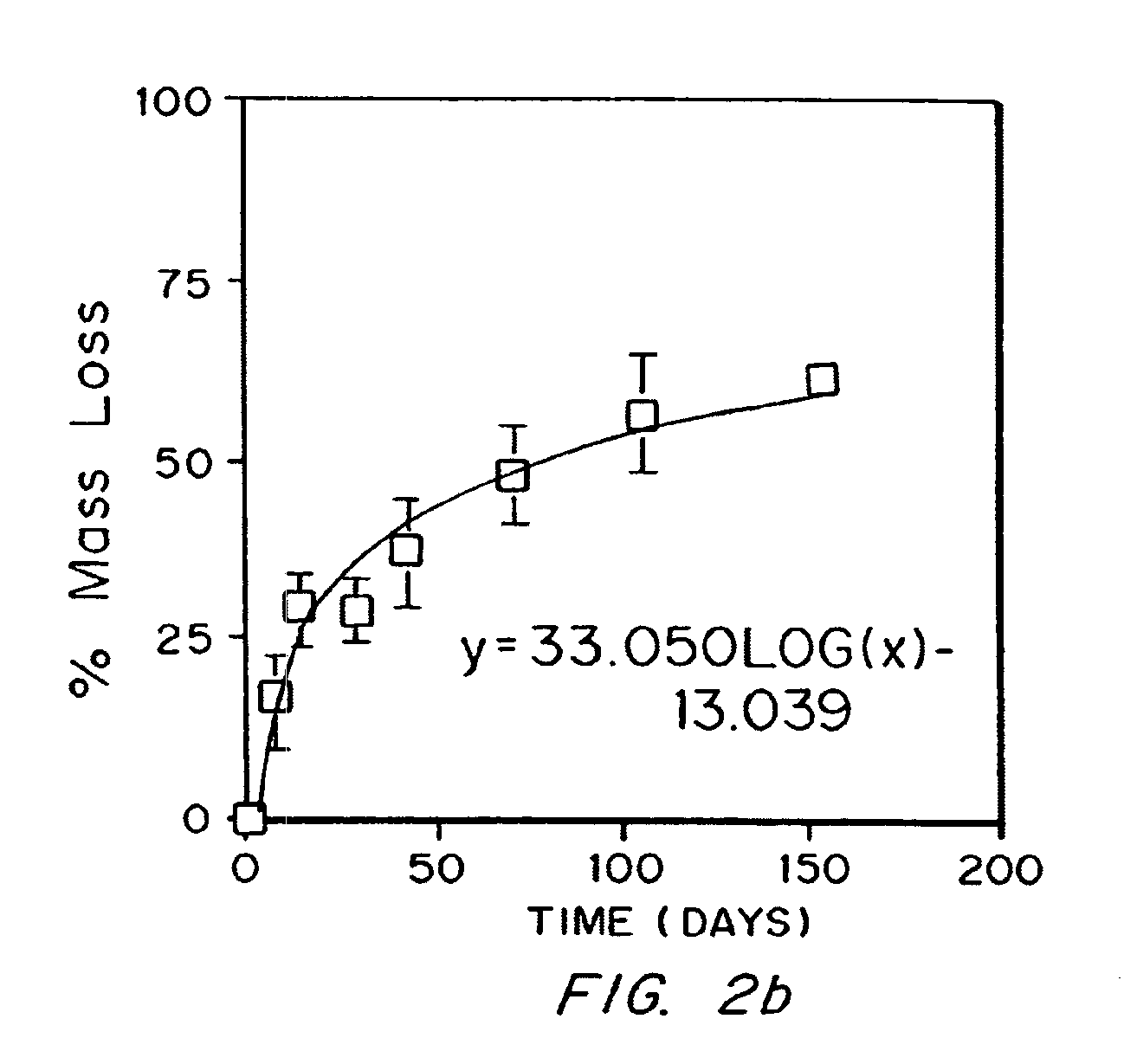
[0130] Seal pressure text on latex to measure strength and elasticity
[0131]Poly(ethylene glycol)-lactide-trimethylene carbonate terpolymers endcapped with acrylate esters were evaluated using a seal pressure test apparatus to determine the failure pressure for coatings prepared using the macromers.
[0132]One of the materials tested had a poly(ethylene glycol) molecular weight of 20,000 Daltons (“20 kD”), a lactate incorporation of 13.8 and a trimethylene carbonate incorporation of 16.0, with nominal acrylation of 2 per macromer (“20KTL”). Also tested were 35 kD PEG esterified with about 8 TMC linkages and then endcapped with acrylates (“35KT”), and 35 kD PEG esterified with about 8 TMC and about 8 lactate groups (“35KTL”), both also acrylated. The reagents applied were “primer” and “sealant”. The complete system contained both a photoinitiation system (Eosin Y / triethanolamine) and a redox initiation system (ferrous gluconate / fructose, plus t-butylhydroperoxide after mixing) which did...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com