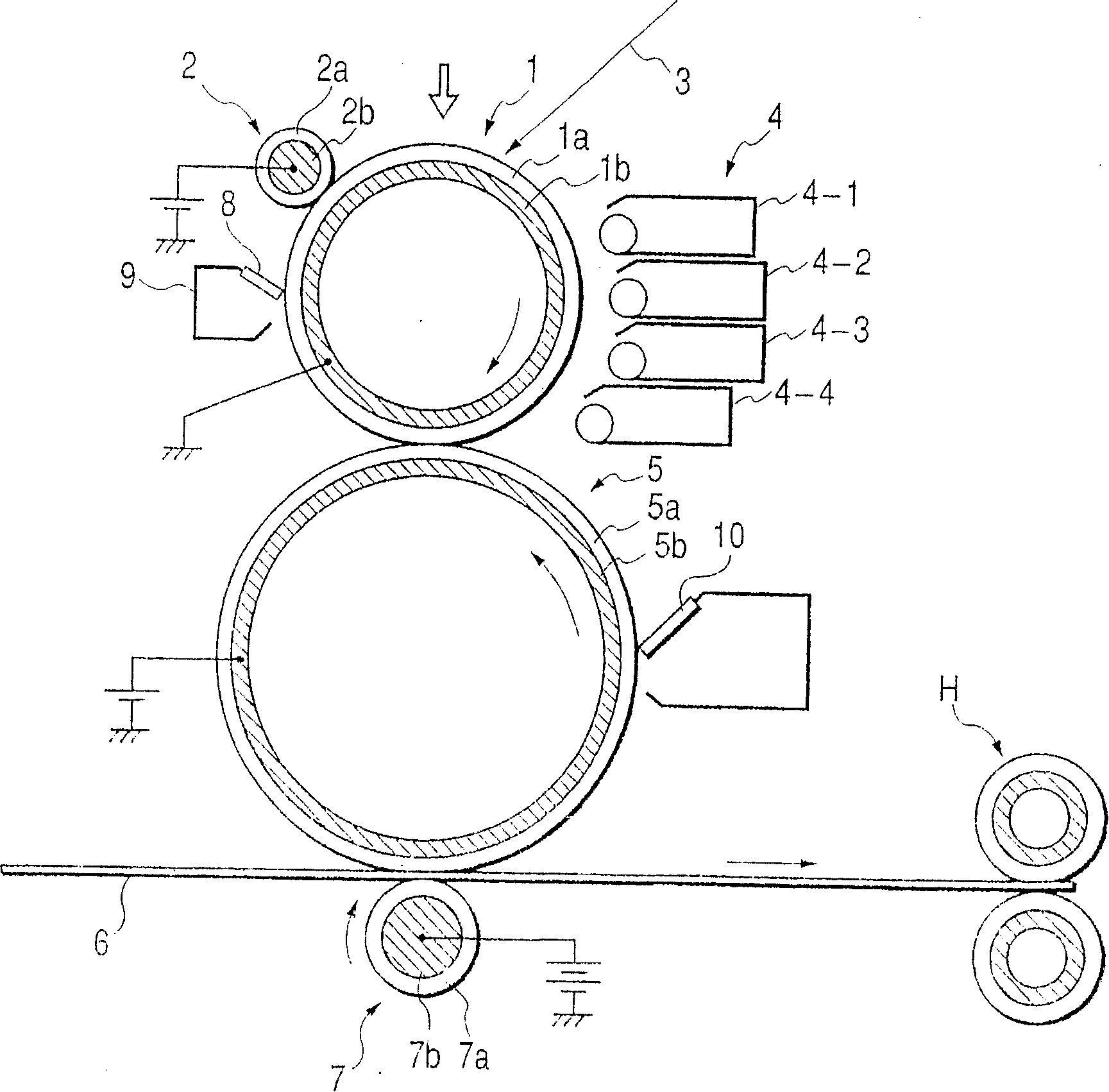
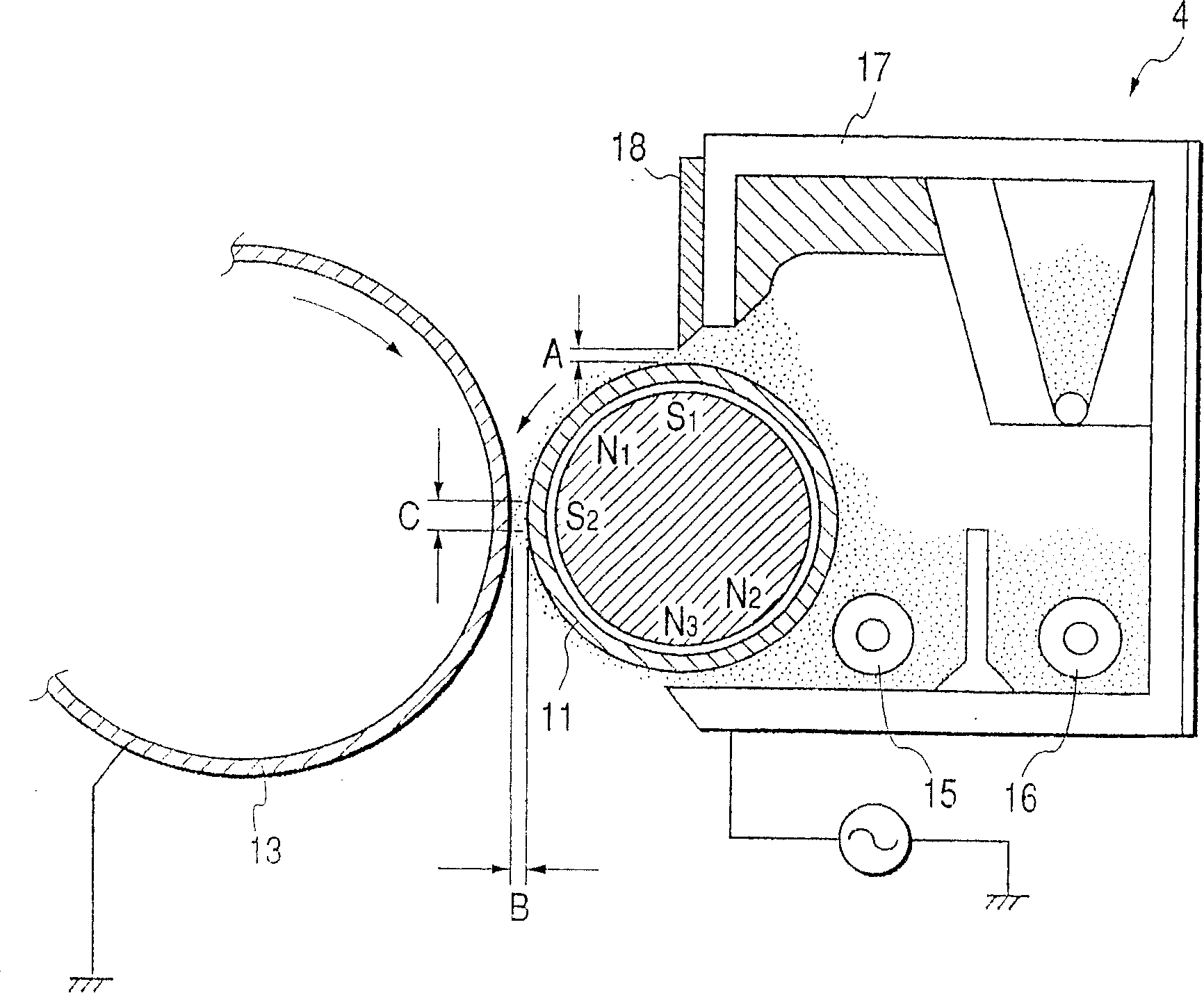
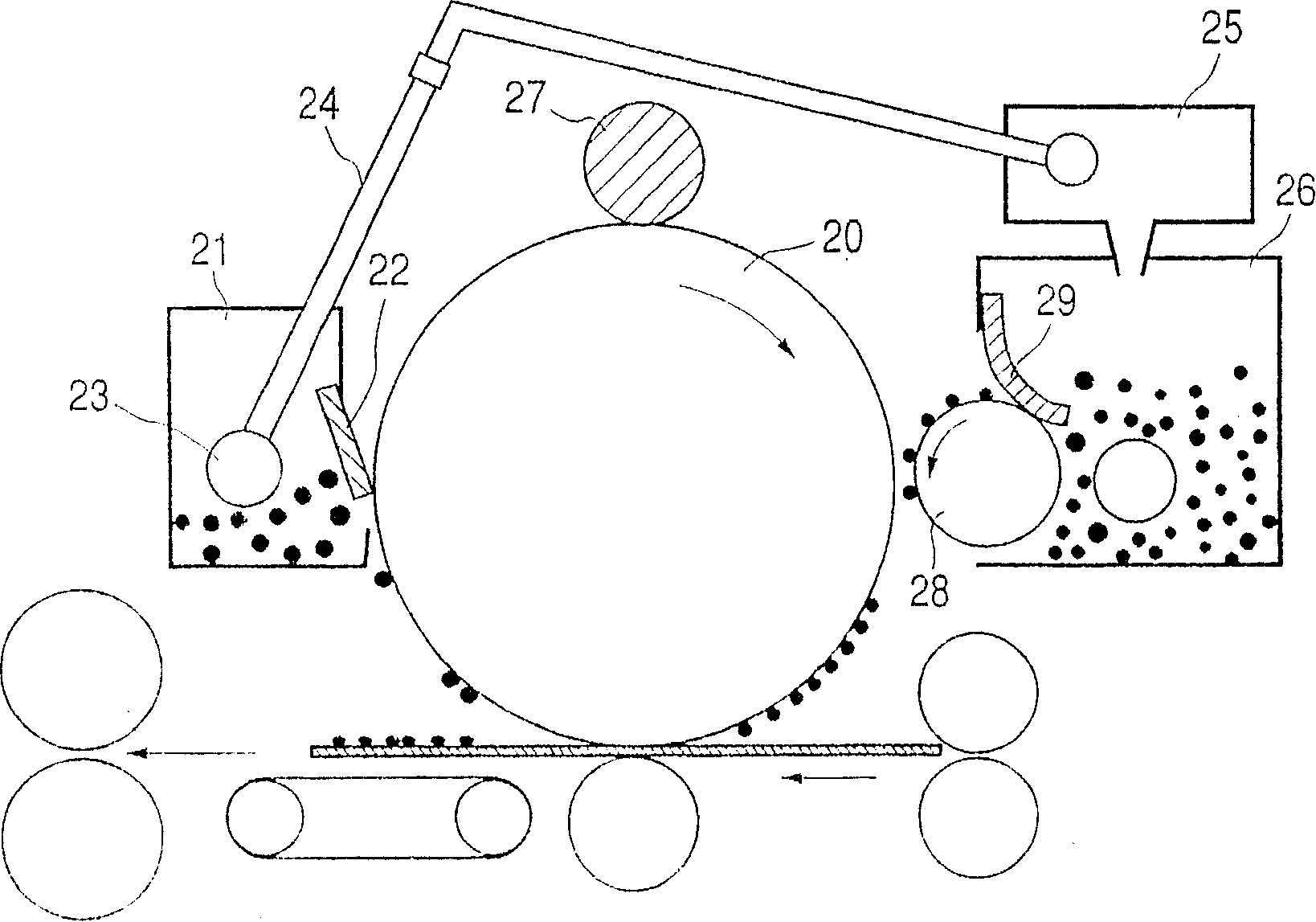
Poly(hydroxy alkanoate), its preparation process, and charge adjusting agent, toner, image forming method, and image forming apparatus
A technology of polyhydroxyalkanoate and methyl, which is applied in the fields of imaging devices, charge regulators, and toners for electrostatic charge image development. It can solve the problems of air pollution, durability, disposal costs, harmful incineration gases, etc. and the effects of improved consumption properties, excellent transferability, and improved melt processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0269] Hereinafter, the present invention will be described in more detail with reference to Examples, but the present invention should not be construed as being limited thereto.
[0270] Reference Example A
[0271] Prepare 20 shake flasks (capacity 500mL), dissolve 0.5wt% polyptone (Wako PureChemical Industries, Ltd.), 6mmol / L 5-phenylpentanoic acid and 2mmol / L 10-undecenoic acid in 200 mL of the above M9 medium, and then place the mixture in individual 500-mL shake flasks. The shake flasks were sterilized in an autoclave and allowed to cool to room temperature. Add culture solution (2 mL) to the obtained culture medium, and in this culture solution, Pseudomonas chicory strain YN2 has been shaken and cultivated in the M9 medium containing 0.5% polyptone in advance for 8 hours, and after the addition, at 30 ° C Incubate for 64 hours. After the cultivation, the respective culture solutions were put together and centrifuged to collect the cells. Cells were washed with metha...
Embodiment A-1
[0296] Under a nitrogen atmosphere, 200 mg of the polymer containing 7 mol% of 3-hydroxy-ω-carboxyalkanoate units obtained in Reference Example A and 22.9 mg of p-toluidine-2-sulfonic acid were placed in a 50-mL double-necked bottle, and added 10mL of pyridine, followed by stirring. Subsequently, 0.22 mL of triphenyl phosphite was added thereto, and the mixture was heated at 100° C. for 6 hours. After the reaction, reprecipitation was performed with EtOH, and the precipitate was recovered by centrifugation. The resulting polymer was washed by stirring in water for 3 days, washed with 1 N hydrochloric acid for an additional day, and dried under vacuum for 1 day.
[0297] The average molecular weight of the resulting PHA was evaluated using gel permeation chromatography (GPC; Tosoh HLC-8020, column; Polymer Laboratories Mixed-C, solvent; DMF containing 0.1% LiBr, equivalent to polystyrene). As a result, the number average molecular weight (Mn) was 28,000.
[0298] Using Fouri...
Embodiment A-2
[0304] The procedure of Example A-1 was repeated except that 27.3 mg of 2-amino-1-naphthalenesulfonic acid was used instead of 22.9 mg of p-toluidine-2-sulfonic acid in Example A-1, thereby obtaining 84 mg of polymer.
[0305] Such as Figure 8 As shown, FT-IR shows the formation of an amide bond due to the decrease of the peak assigned to the carboxylic acid and the appearance of a new peak assigned to the amide group. The resulting PHA was found to contain a unit represented by the chemical formula (8A).
[0306] The average molecular weight of the obtained PHA was analyzed in a similar manner to Example A-1. The number average molecular weight (Mn) of PHA was 27,500. Also, from the measurement results of the amount of sulfur, it was found that the obtained PHA was a PHA copolymer containing 4.7 mol% of 2-amino-1-naphthalenesulfonic acid groups.
[0307] 50 g of a PHA copolymer was obtained by a large-scale production method, and this compound was used as Exemplary Compou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com