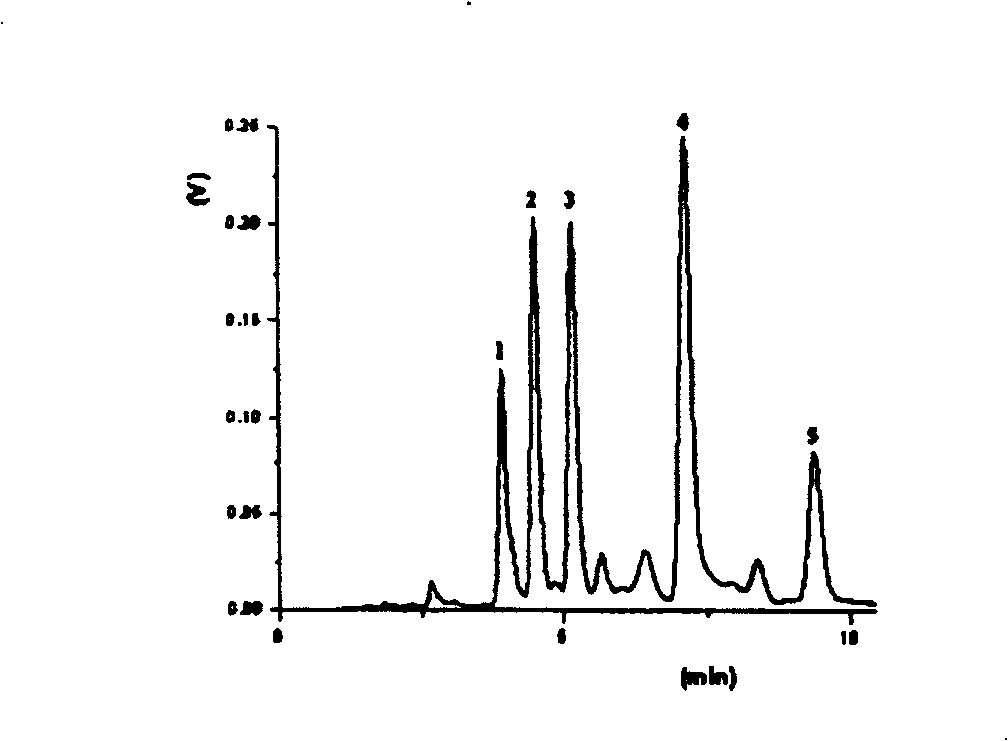
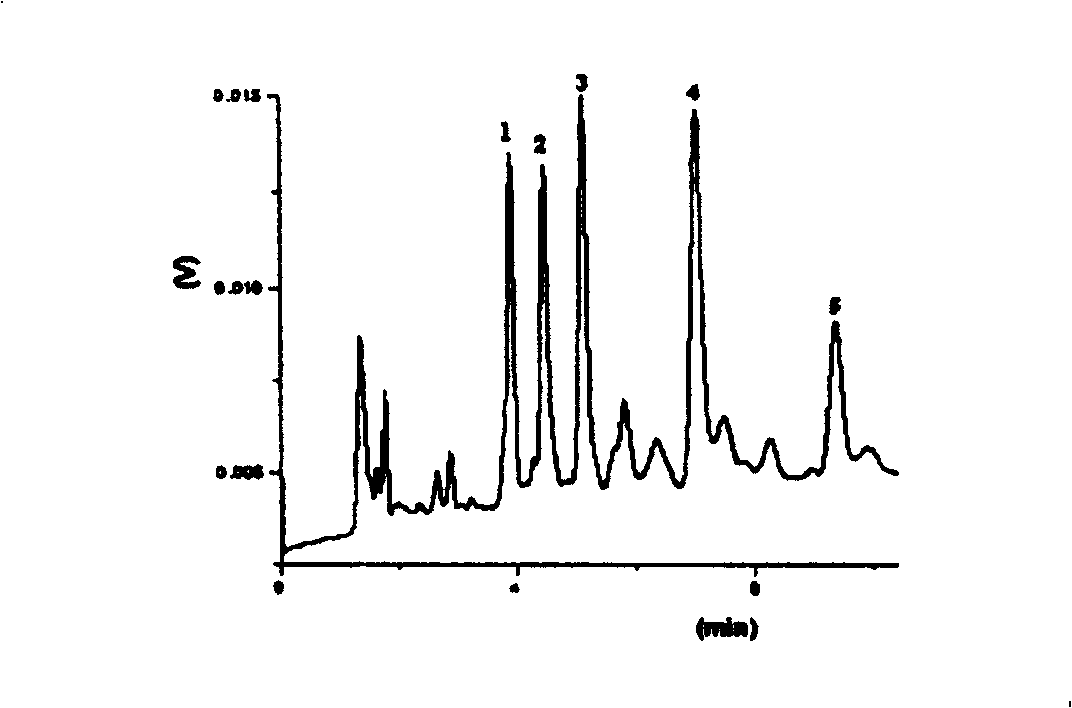
Preparation method of-2-(N-carbazolyl)-ethoxy carbohydrazide
An ethoxycarbazide and carbazole-based technology is applied in the field of preparation of 2-ethoxycarbazide, and can solve the problems of low component detection sensitivity, high fluorescence quantum efficiency, unstable derivative products, and the like, Achieve the effects of fast derivatization, high fluorescence quantum efficiency, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Add 30g of carbazole and 150mL of dimethyl sulfoxide into a 500ml round bottom flask, start electromagnetic stirring and heat, add 10g of potassium hydroxide in batches within 20 minutes, keep the temperature of the solution at 100°C and reflux and stir for 30 minutes, and 40 g of 2-bromoethanol was added within 1 hour, and the temperature was kept at 100° C., and the reaction was continued under reflux and stirring for 2.5 hours, and the solution gradually turned dark brown. Cool the mixture in the flask to room temperature. After the reaction is complete, use a rotary evaporator to evaporate the solvent under the condition of heating an oil bath. The residue is washed with water three times, dried and heated to boiling with n-hexane for extraction. After cooling, a white solid is precipitated. , After suction filtration, the filter cake was recrystallized twice with n-hexane to obtain white needle-like crystals.
[0021] 15g of phosgene is dissolved in 100ml of methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com