Method for preparing pollen pini polyose, pollen pipe polyose and its use in medicine
A technology of pine pollen and polysaccharides, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve the effects of improving immunity, improving blood sugar and blood pressure, and lowering blood sugar and blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of pine pollen polysaccharide
[0027] (1) Extraction: Take 2kg of broken-walled pine pollen, add 60L of water, stir and heat up to 95°C-100°C in a heating extraction tank, stir and extract at a constant temperature for 8 hours, and drop to 40-45°C.
[0028] (2) Impurity removal: adjust the pH to 8.0-8.5 with NaOH, add trypsin with 3% weight of pine pollen, incubate at 40-45°C for 4 hours, then heat up to 95°C, maintain for 15 minutes, and then lower to room temperature Adjust the pH to 5.5-6.0 with HCl, add cellulase with 3% weight of pine pollen, incubate at 40-45°C for 4 hours, heat up to 95°C, maintain it for 15 minutes, and cool down to room temperature. Centrifuge at a high speed with a relative centrifugal force of 9100g, collect the supernatant, and ultrafilter with an ultrafiltration membrane with a molecular weight cut-off of 100kd to collect the non-filtrate.
[0029] (3) Alcohol precipitation: the non-filtrate was heated and co...
Embodiment 2
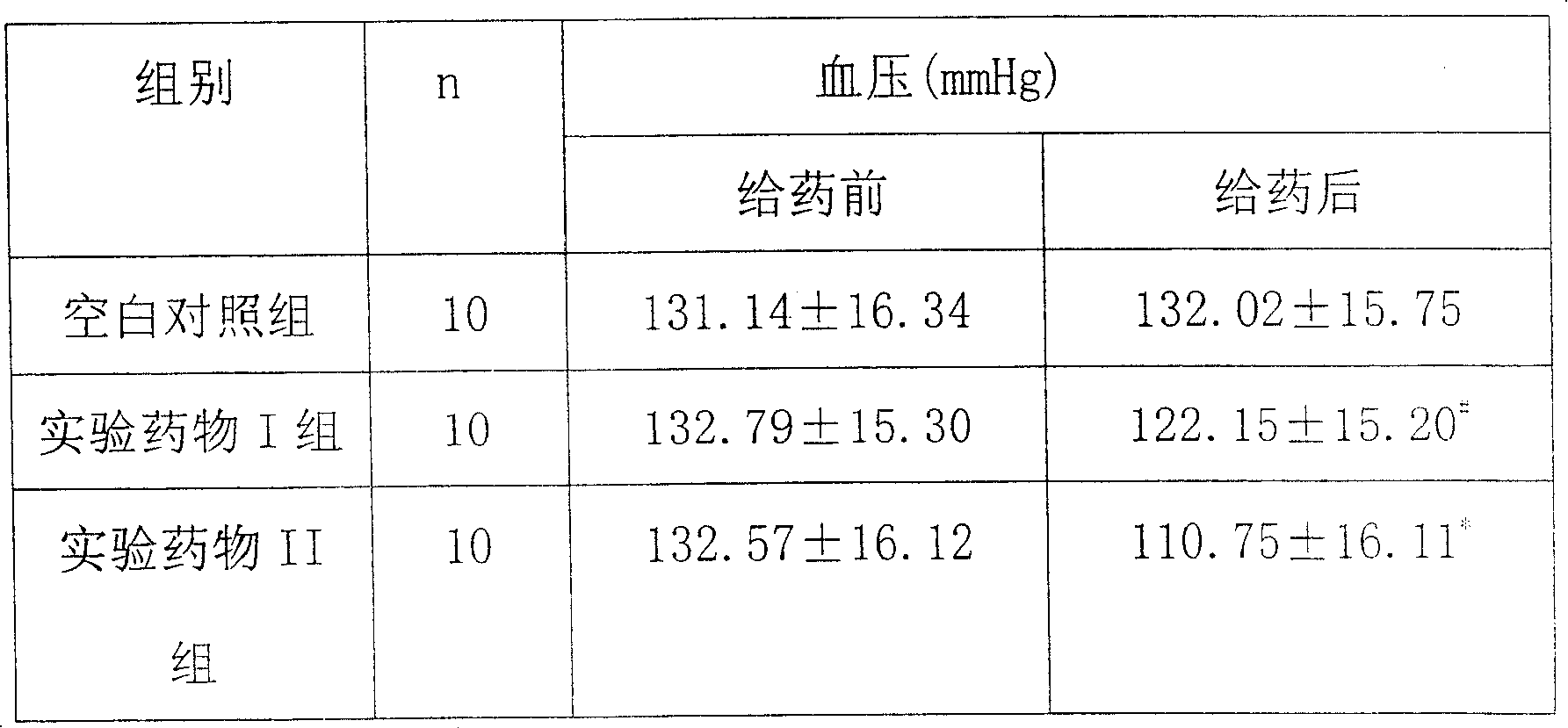
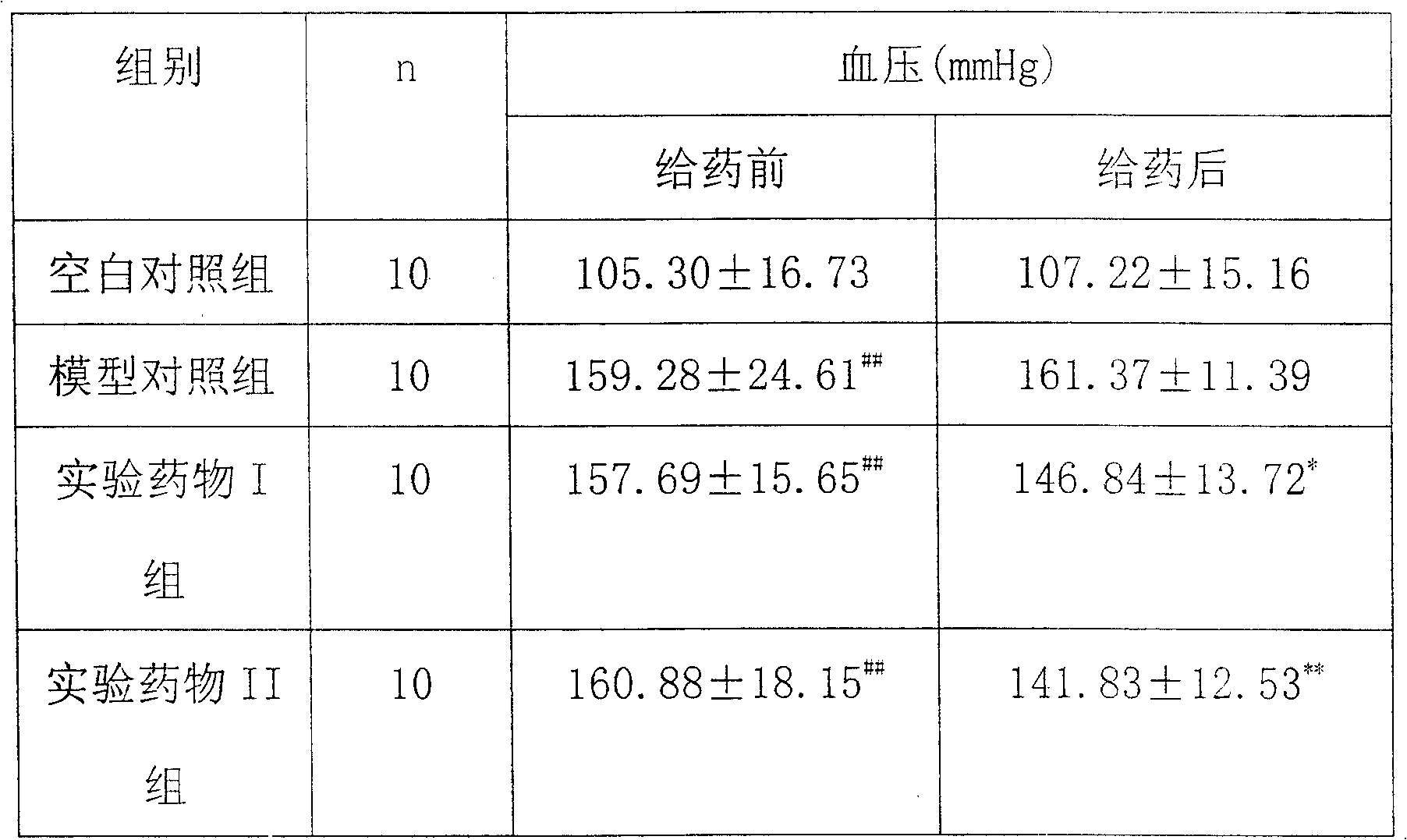
[0032] Example 2: Effect of pine pollen polysaccharide on blood pressure level in renally hypertensive rats
[0033] 1. Experimental animals and group settings
[0034] Wistar rats, 9-10 weeks old, weighing 200±20g, male, were randomly divided into a blank control group, a model control group, and experimental drug groups I and II, with 10 rats in each group.
[0035] 2. Preparation of hypertensive rat model
[0036] Use diazepam 5mg / kg and ketamine 50mg / kg, intradermally anesthetize rats, fix in supine position, after skin disinfection, incise the skin and muscle layer along the midline of the abdomen at 1.5cm below the xiphoid process, gently squeeze out the kidney, Wrap it with saline cotton and fix it with your left index finger and thumb. Use the right hand to carefully separate the fascia of the renal pedicle with straight ophthalmic tweezers without hooks, separate the renal artery along the lower part of the renal vein, wrap it with an H-shaped silver clip (0.20-0.25...
Embodiment 3
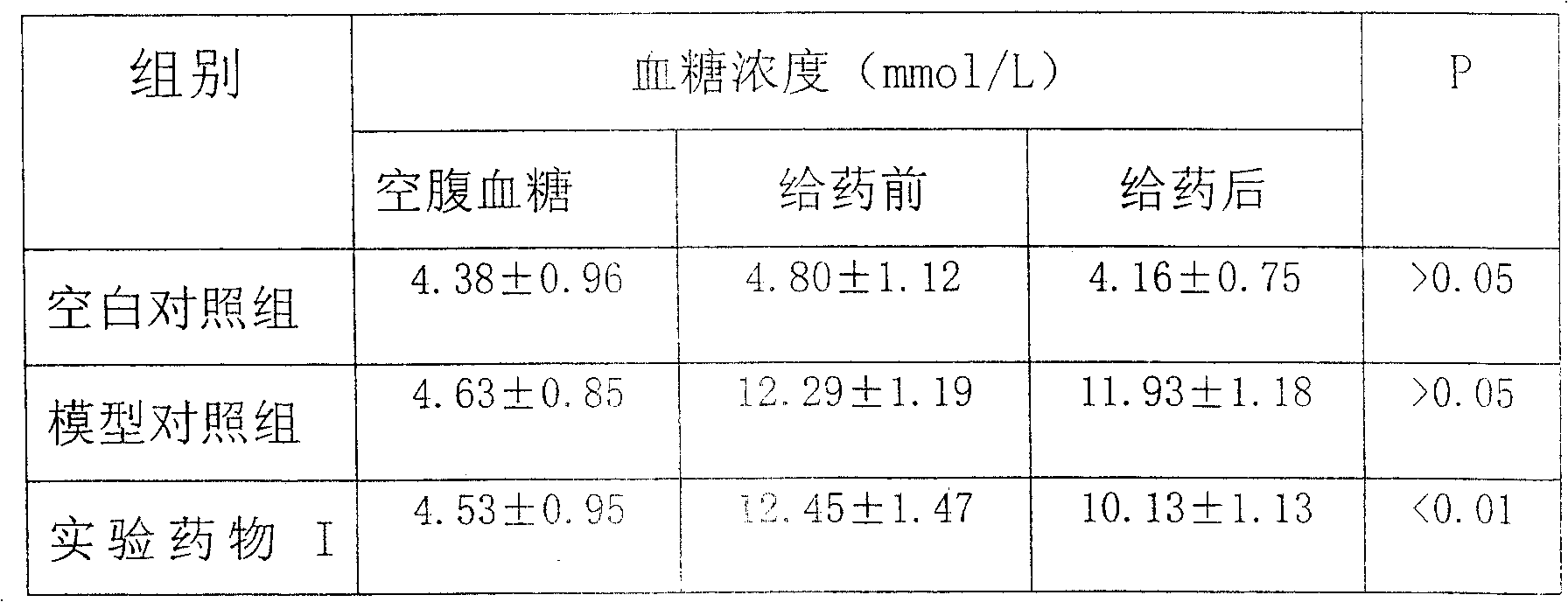
[0053] Example 3: Effect of pine pollen polysaccharide on blood lipid levels in rats with dyslipidemia
[0054] 1. Animal selection and group setting
[0055] With embodiment 2.
[0056] 2. Rat hyperlipidemia model preparation
[0057] Rat hyperlipidemia model adopts the method of hyperlipidemia induced by high-fat diet. The formula of high-fat feed is as follows: 86.3% of basic feed, 3% of cholesterol, 10% of lard, 0.2% of methylthiouracil, and 0.5% of pig bile salt, so as to ensure that the ingredients are mixed evenly. Continuous feeding for 2 weeks, anesthetized with pentobarbital sodium solution, tail blood was taken, and blood lipids were detected, which proved that the model was successfully manufactured.
[0058] 3. Experimental method
[0059] Blank control group: no modeling, no drug administration, and an equal amount of normal saline;
[0060] Model control group: modeling, no drug administration, and high-fat feed every morning;
[0061] Experimental drug gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com