Method for preparing steroid muscular relaxant and analogue thereof
A compound and steroid technology, applied in the field of preparation of steroid muscle relaxants and similar compounds, can solve the problems of low yield and the like, and achieve the effects of good selectivity, shortened reaction time, and reduced hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
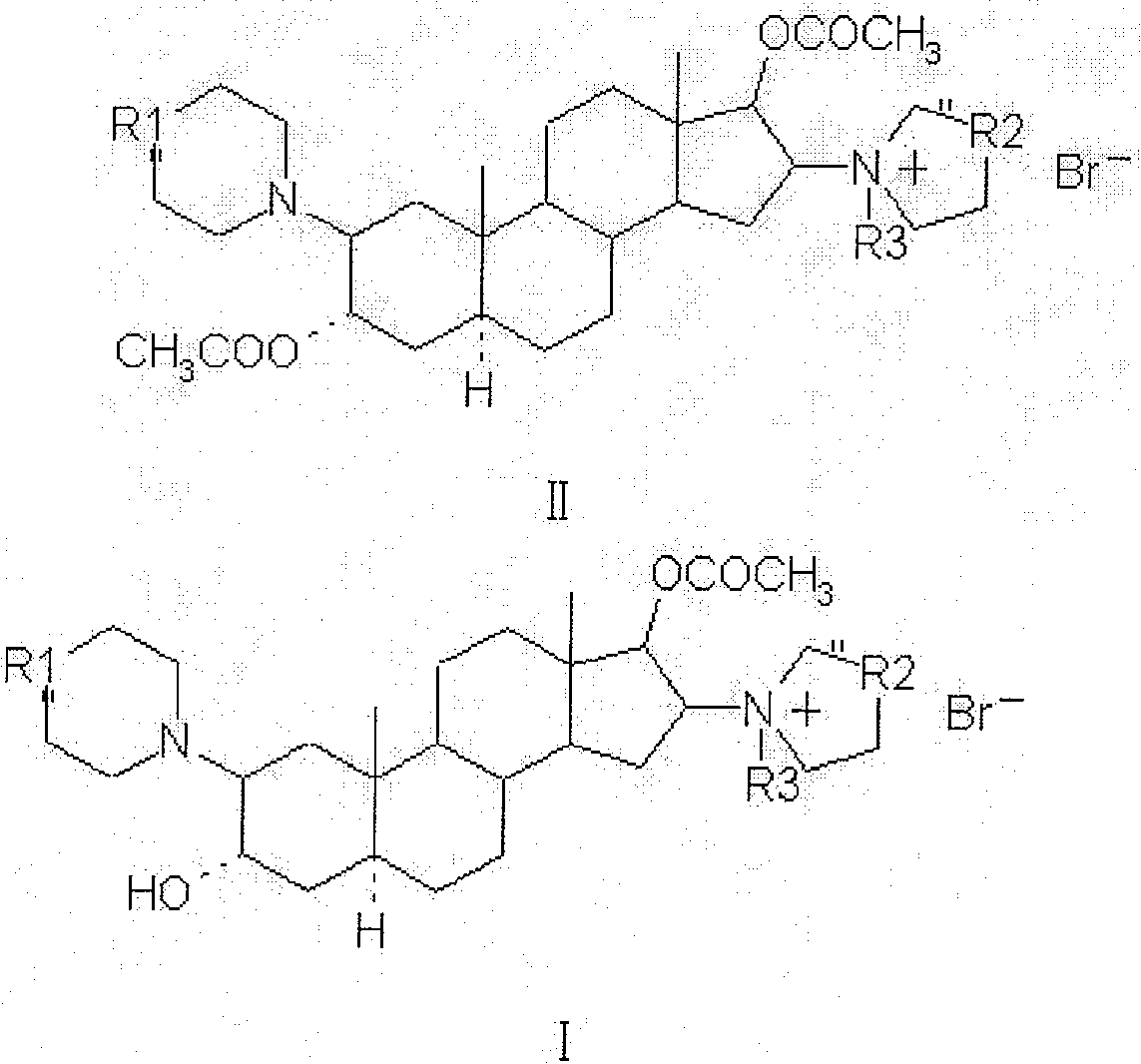
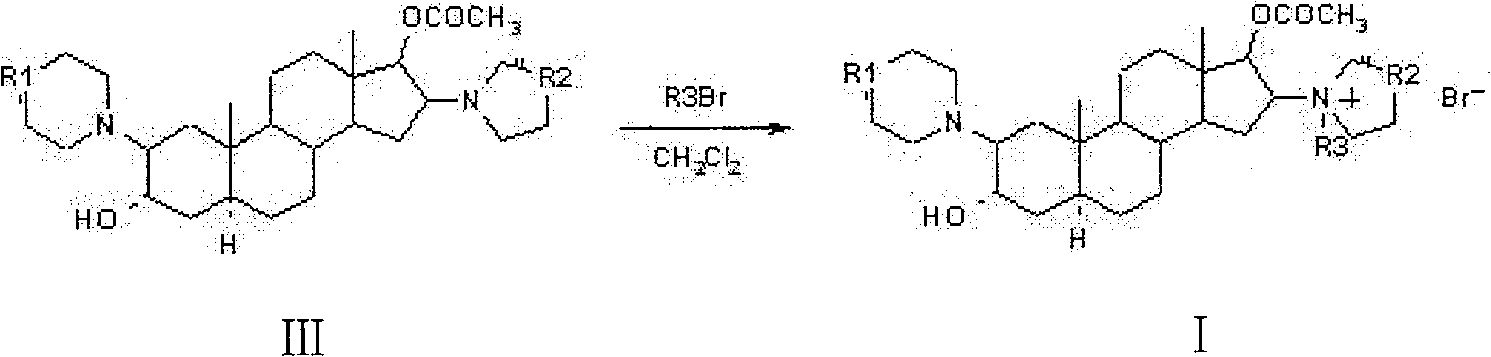
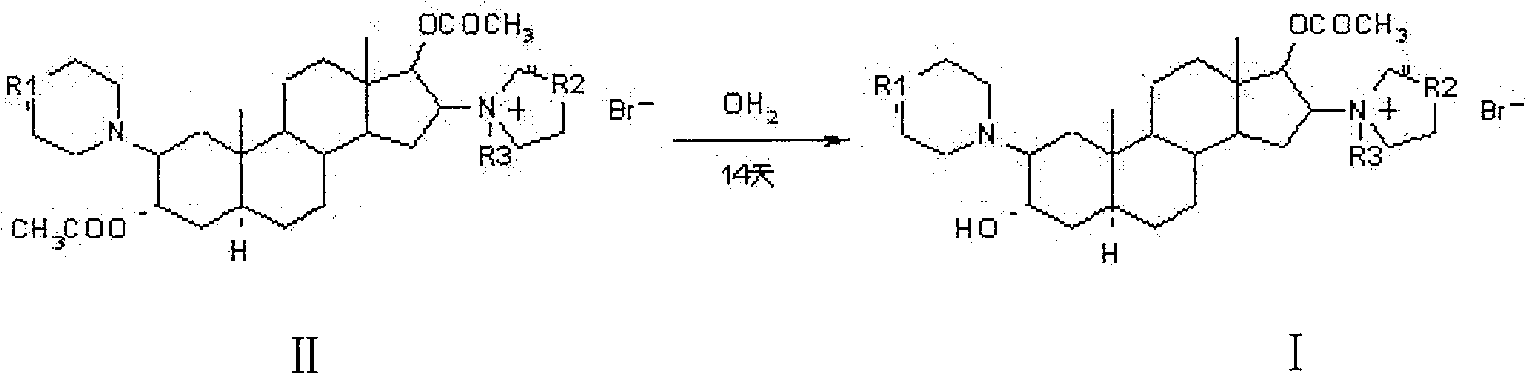
[0028] Example 1: 1-[17β-Acetoxy-3α-hydroxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (rocuronium bromide) preparation
[0029] 3.0g 1-[3α, 17β-diacetoxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (acetyl Cucuronium bromide) was dissolved in 30ml of dichloromethane, added 9ml of 18% hydrobromic acid, stirred, heated, and reacted at 40°C for 4.5 hours; then, cooled to 2°C, added pre-cooled 5-10% bicarbonate Neutralize with sodium aqueous solution to make the PH value 7-8, let it stand in a timely manner, and separate layers; the water layer is extracted with dichloromethane several times to extract the product, and the extracted solutions are combined, and dehydrated with anhydrous sodium sulfate to clean. Concentrate to dryness under reduced pressure below 40°C to obtain 2.2 g of rocuronium bromide, with an HPLC percent area purity of 98.7%.
Embodiment 2
[0030] Example 2: 1-[17β-Acetoxy-3α-hydroxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (rocuronium bromide) preparation
[0031] 3.0g 1-[3α, 17β-diacetoxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (acetyl Cucuronium bromide) was dissolved in 9ml of 9% hydrobromic acid, and reacted at 25°C for 15 hours under stirring; then, 30ml of dichloromethane was added, cooled to 2°C, and neutralized by adding pre-cooled 2-5% ammonia solution , so that the pH value is 7-8, let it stand at the right time, and separate layers; the water layer is extracted with dichloromethane several times to extract the product, and the extraction solution is combined, and dehydrated with anhydrous sodium sulfate to the net. Concentrate to dryness under reduced pressure below 40°C to obtain 1.95 g of rocuronium bromide, with an HPLC percent area purity of 98.5%.
Embodiment 3
[0032] Example 3: 1-[17β-Acetoxy-3α-hydroxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (rocuronium bromide) preparation
[0033] 3.0 g of 1-[3α, 17β-diacetoxy-2β-(4-morpholinyl)-5α-androstane-16β-yl]-1-(2-propenyl)pyrrolidine bromide (acetyl Rocuronium bromide) was dissolved in 30ml of acetone, 15ml of 27% hydrobromic acid was added, heated under stirring, and reacted at 50°C for 2 hours; then, 30ml of chloroform was added, cooled to 5°C, and pre-cooled 10 ~15% potassium bicarbonate aqueous solution is neutralized, so that the pH value is 7~8, it is allowed to stand in a timely manner, and the layers are separated; the water layer is extracted with chloroform several times to extract the product, and the extracted solution is combined, and dehydrated with anhydrous sodium sulfate to the net. Concentrate to dryness under reduced pressure below 40°C to obtain 2.1 g of rocuronium bromide, with an HPLC percent area purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com