Stable elaioplast composition
A liposome composition and liposome technology are applied in the direction of liposome delivery, drug combination, medical preparations of non-active ingredients, etc., which can solve the problems of not always being reliable, so as to improve tolerance and avoid Hypersensitivity, stable quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 paclitaxel liposome
[0056] prescription:
[0057] Paclitaxel 0.37g
[0058] Hydrogenated Soy Lecithin 4g
[0059] Cholesterol 1g
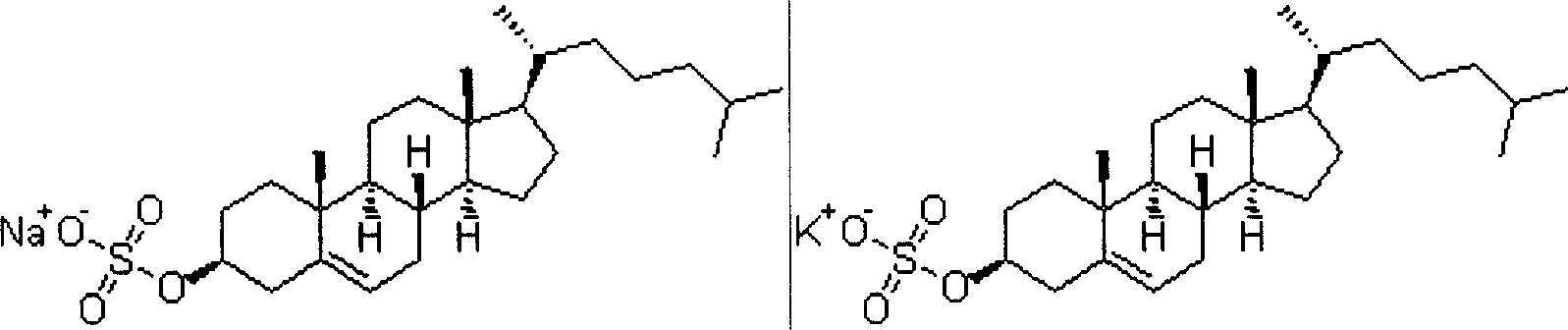
[0060] Sodium Cholesterol Sulfate 0.05g
[0061] Vitamin E 0.055g
[0062] Sucrose 15g
[0063] Water for injection 120ml
[0064] Weigh the prescribed amount of paclitaxel, hydrogenated soybean lecithin, cholesterol, sodium cholesteryl sulfate, vitamin E and add 100ml of absolute ethanol, after dissolving, dry the solvent with a rotary evaporator in the presence of nitrogen, prepare a film, and put it in a vacuum Place in a desiccator overnight to remove residual solvent. Add sucrose solution dissolved in water for injection, 55°C water bath, magnetically stir for 1 hour, ultrasonically disperse, homogenize with a high-pressure homogenizer, filter aseptically with a 0.22-micron PVDF membrane, divide into vials, and freeze-dry. Ventilate the vial with nitrogen, and seal it with a gland.
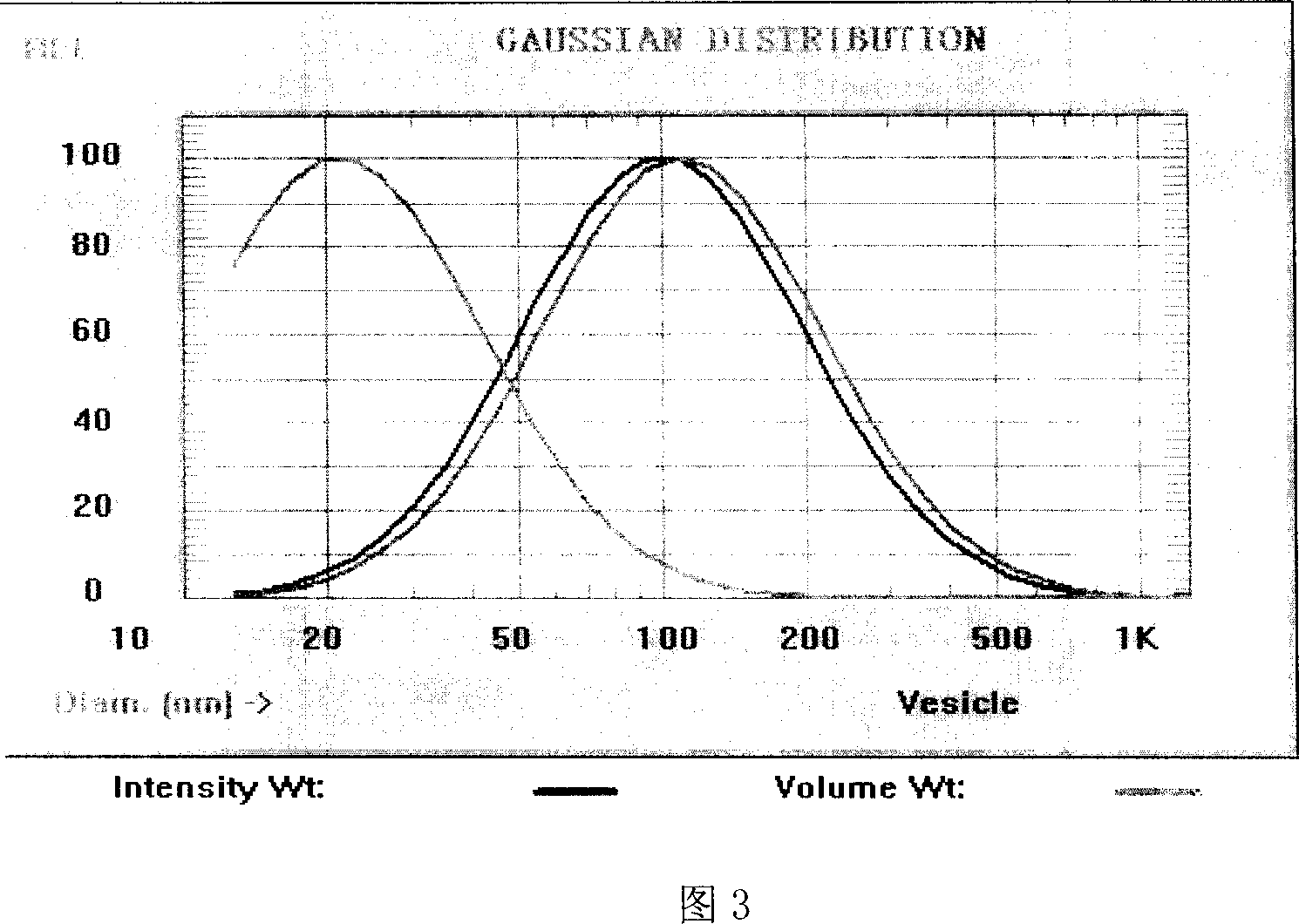
[0065] Acc...
Embodiment 2
[0067] The preparation of embodiment 2 paclitaxel liposomes
[0068] prescription:
[0069] Paclitaxel 0.09g
[0070] Hydrogenated Soy Lecithin 4g
[0071] Cholesterol 0.4g
[0072] Potassium Cholesterol Sulfate 0.006g
[0073] Vitamin E 0.012g
[0074] Lactose 10g
[0075] Water for injection 150ml
[0076] Respectively weigh paclitaxel, hydrogenated soybean lecithin, cholesterol, potassium cholesteryl sulfate, vitamin E and add 100ml of absolute ethanol to dissolve, prepare a film with a rotary evaporator, and place it in a vacuum desiccator overnight to remove the residual solvent. Add sucrose solution dissolved in water for injection, 58 ° C water bath, magnetic stirring for 1 hour, ultrasonic dispersion, sequentially use 0.8, 0.4, 0.2 micron polycarbonate film, extrude with extruder under high pressure, divide into vials, freeze dry , gland seal.
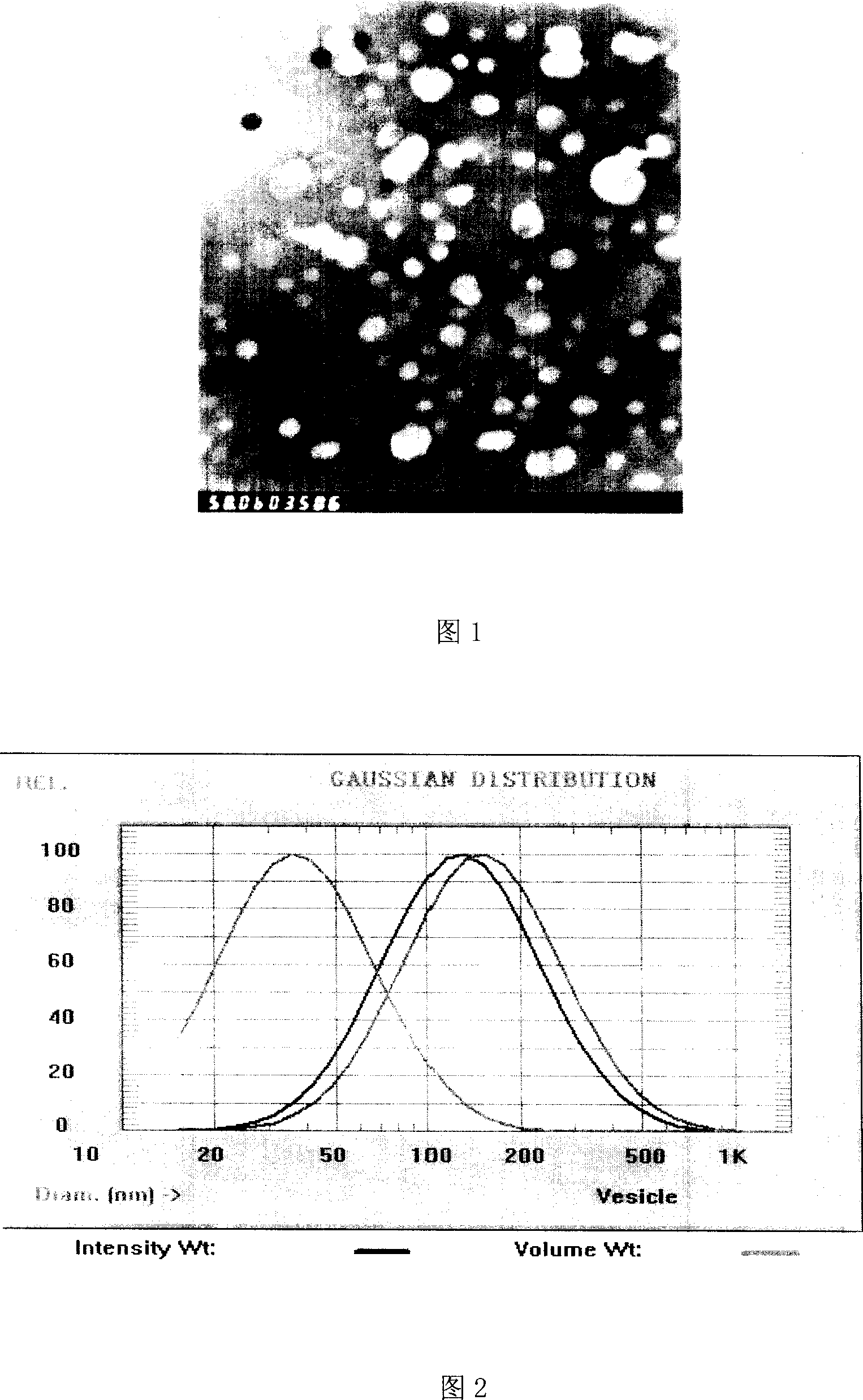
[0077] Measured in the same manner as in Example 1, the average particle diameter of the sample before freeze-drying ...
Embodiment 3
[0078] The preparation of embodiment 3 paclitaxel liposomes
[0079] prescription:
[0080] Paclitaxel 0.18g
[0081] Hydrogenated Soy Lecithin 4g
[0082] Cholesterol 0.9g
[0083] Sodium Cholesterol Sulfate 0.13g
[0084]Vitamin E 0.04g
[0085] Sucrose 25g
[0086] Water for injection 150ml
[0087] Weigh paclitaxel, hydrogenated soybean lecithin, cholesterol, sodium cholesteryl sulfate, vitamin E and add 100ml of absolute ethanol respectively, after dissolving, prepare a film with a rotary evaporator, place it in a vacuum desiccator overnight, and remove the residual solvent. Add sucrose solution dissolved in water for injection, 60°C water bath, magnetically stir for 1 hour, ultrasonically disperse, homogenize with a high-pressure homogenizer, extrude with a 0.2-micron polycarbonate membrane, filter aseptically with a 0.22-micron PVDF membrane, and dispense into Vials, freeze-dried, sealed with a gland.
[0088] Measured in the same manner as in Example 1, the ave...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com