Compound of containing nitrine triazine symmetrel and synthetic method
A technology of azidotriazine amantadine and a synthesis method, which is applied in the field of compounds containing azide triazine amantadine and its synthesis, can solve the problems of reducing the sensitivity of new compounds, poor thermal stability, reducing costs, etc. Safe production and use, the effect of reducing sensitivity and changing molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
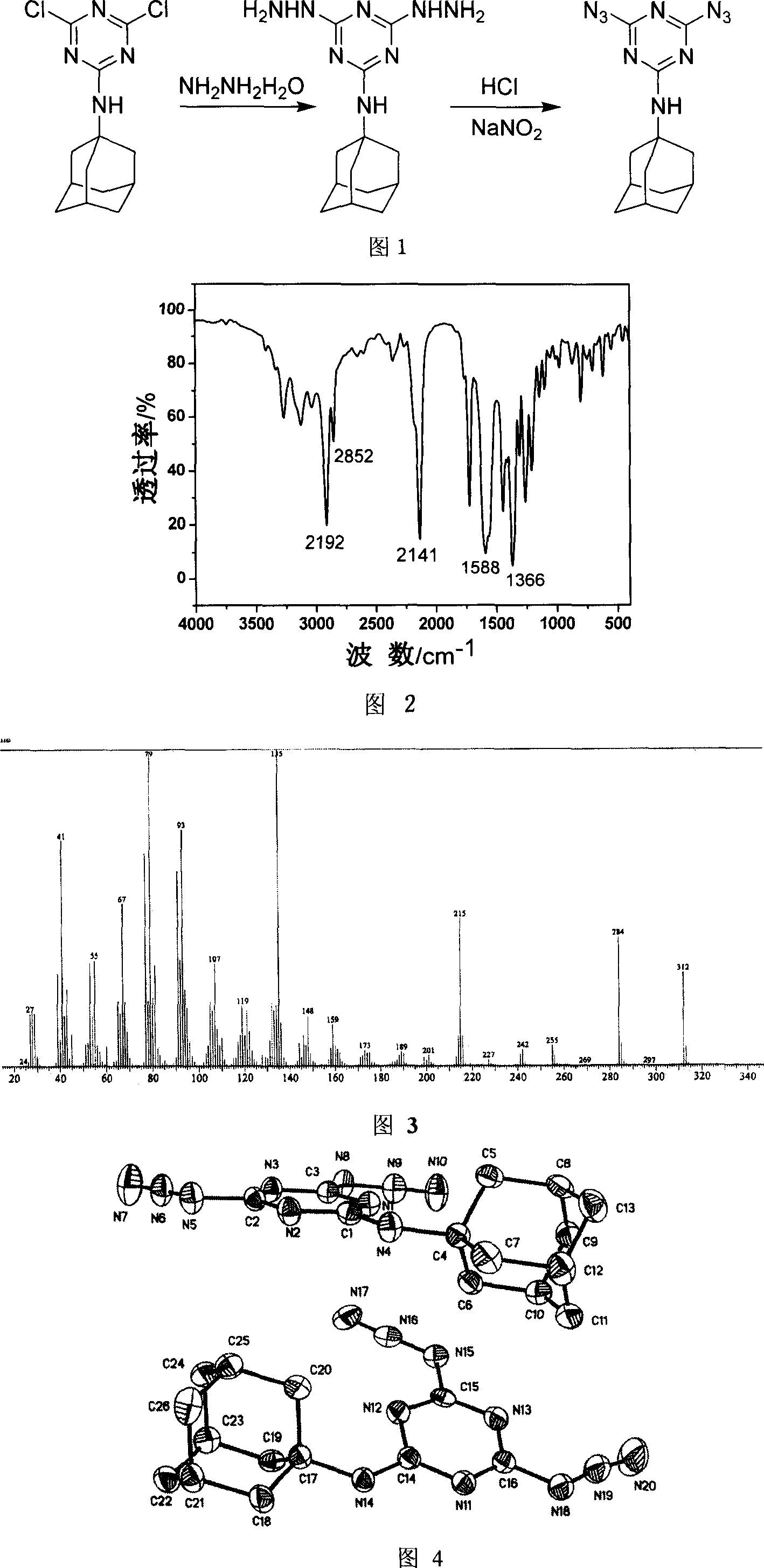
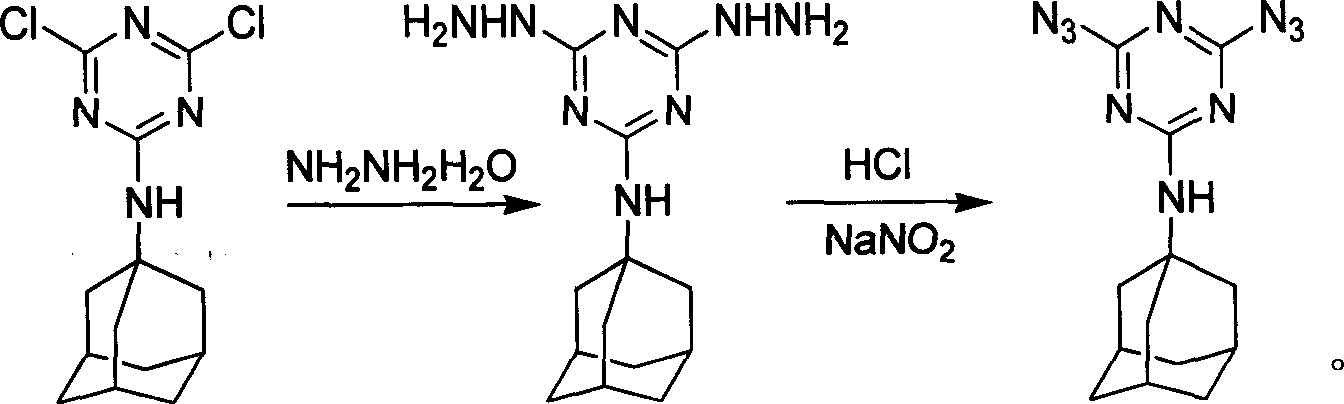
[0029] Synthetic method of diazidetriazine amantadine, see accompanying drawing 1 for the synthetic route, in the first step, in 100mL acetonitrile, add 0.5mol dichlorotriazine amantadine and 5mL of hydrazine hydrate with a concentration of 80%, at 80°C After heating and stirring for 5 hours, the reaction was stopped, cooled to 20°C, and dried by filtration. In the second step, in 10 mL of 1 mol / L hydrochloric acid solution, add 1 mmol of dihydrazinotrinitrobenzamantadine at a reaction temperature of 0°C, add sodium nitrate dropwise, and stop adding sodium nitrite when the starch potassium iodide test paper changes color , the product was extracted three times with 20 mL of ethyl acetate, concentrated by rotary evaporation, and dried in a water-bath oven at 50°C for more than 1 hour to obtain a dry white product, which was dissolved in ethyl acetate and volatilized slowly to obtain a single crystal of the product. The yield 80% (based on amantadine dichlorotriazine). For the ...
Embodiment 2
[0031] Synthetic method of diazidetriazine amantadine, see accompanying drawing 1 for the synthetic route, in the first step, in 100mL acetone, add 0.5mol dichlorotriazine amantadine and 5mL of hydrazine hydrate with a concentration of 80%, at 50°C After heating and stirring for 7 hours, the reaction was stopped, cooled to 20°C, and dried by filtration. In the second step, in 10 mL of 1 mol / L hydrochloric acid solution, add 1 mmol of dihydrazinotrinitrobenzamantadine at a reaction temperature of 0°C, add sodium nitrate dropwise, and stop adding sodium nitrite when the starch potassium iodide test paper changes color , the product was extracted three times with 20 mL of ethyl acetate, concentrated by rotary evaporation, and dried in a water-bath oven at 50°C for more than 1 hour to obtain a dry white product, which was dissolved in ethyl acetate and volatilized slowly to obtain a single crystal of the product. The yield More than 76% (based on dichlorotriazine amantadine). For...
Embodiment 3
[0033] Synthetic method of diazidetriazine amantadine, the synthetic route is shown in accompanying drawing 1, the first step is in 100mL tetrahydrofuran, add 0.5mol dichlorotriazine amantadine and the concentration is 80% hydrazine hydrate 5mL, at 78 ℃ After heating and stirring for 8 hours, the reaction was stopped, cooled to 20°C, and dried by filtration. In the second step, in 10 mL of 1 mol / L hydrochloric acid solution, add 1 mmol of dihydrazinotrinitrobenzamantadine at a reaction temperature of 0°C, add sodium nitrate dropwise, and stop adding sodium nitrite when the starch potassium iodide test paper changes color , the product was extracted three times with 20 mL of ethyl acetate, concentrated by rotary evaporation, and dried in a water-bath oven at 50°C for more than 1 hour to obtain a dry white product, which was dissolved in ethyl acetate and volatilized slowly to obtain a single crystal of the product. The yield 75% (based on amantadine dichlorotriazine). For the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com