Prepn of nanometer carbon material supported metal catalyst for hydrogenating chloronitrobenzene to synthesize chloroaniline
A technology of chloronitrobenzene and chloroaniline, which is applied in the field of catalysts for the synthesis of chloroaniline by hydrogenation of chloronitrobenzene, which can solve the problems of poor dispersion of catalyst active components, uneven metal particle size, and catalyst agglomeration and other problems, to achieve the effect of excellent reaction performance, short preparation cycle and low dechlorination performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Carbon nanofiber or carbon nanotube activation treatment:
[0017] Put carbon nanofibers or carbon nanotubes into a round bottom flask, add a mixed solution of sulfuric acid and nitric acid with a volume ratio of 3:1, reflux at 120°C for 4 hours, filter, wash with water until the filtrate is neutral, and dry in air at 100°C for 12 Hour.
[0018] (2 Preparation of carbon nanofiber supported metal nickel catalyst
[0019] Weigh 0.212g Ni(AC) 2 Add 0.5g mixed acid-treated carbon nanofibers into 50mL ethylene glycol, ultrasonically disperse for 5 minutes, then raise the temperature to 80°C, add 0.9mL 0.2M Na 2 CO 3 The aqueous solution was filtered after constant temperature for 1 hour, washed with water, and dried to obtain the catalyst precursor. Put the obtained precursor in a tubular heating furnace, raise the temperature to 400°C at a rate of 3°C / min in a high-purity nitrogen atmosphere, keep it warm for 4 hours, then switch to a mixed gas of nitrogen and hydro...
Embodiment 2
[0023] (1) The carbon nanofiber activation treatment is the same as in Example 1.
[0024] (2) Preparation of carbon nanofiber-supported metal nickel catalyst
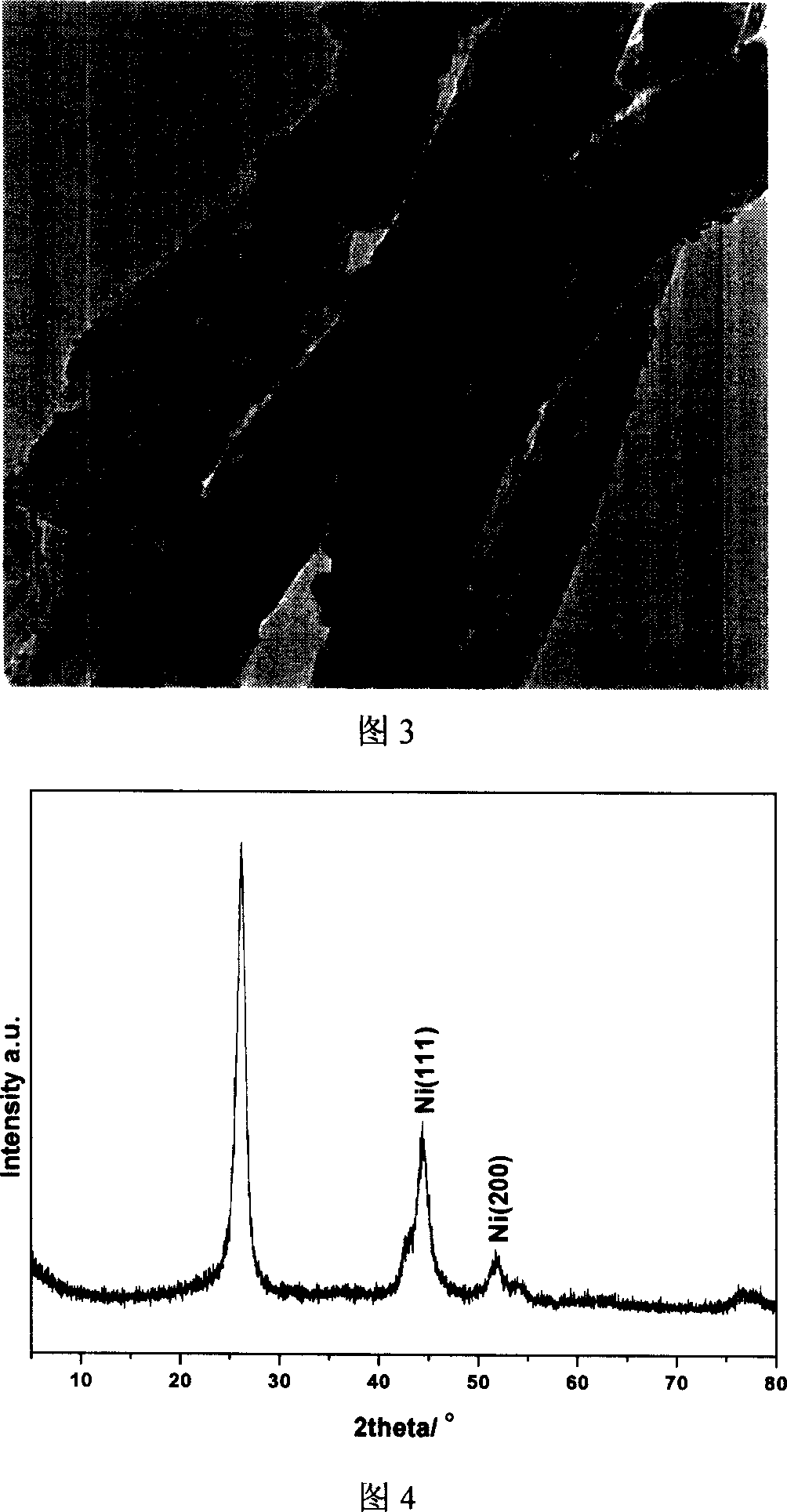
[0025] Weigh 0.212g Ni(AC) 2 Add 0.5g mixed acid-treated carbon nanofibers into 50mL ethylene glycol, ultrasonically disperse for 5 minutes, then raise the temperature to 120°C, add 0.9mL 0.2M Na 2 CO 3 The aqueous solution was filtered after constant temperature for 1 hour, washed with water, and dried to obtain the catalyst precursor. The obtained precursor was placed in a tubular heating furnace, and the temperature was raised to 400°C at 3°C / min in a high-purity nitrogen atmosphere, kept for 4 hours, and then switched to nitrogen and hydrogen mixed gas for reduction for 1 hour to obtain carbon nanofiber-supported metal nickel catalyst. ICP results showed that the mass percent content of metallic nickel was 6.0%. The XRD calculation results show that the average particle size of metallic nickel particles is 5.1...
Embodiment 3
[0028] (1) The carbon nanofiber activation treatment is the same as in Example 1.
[0029] (2) Preparation of carbon nanofiber-supported metal nickel catalyst
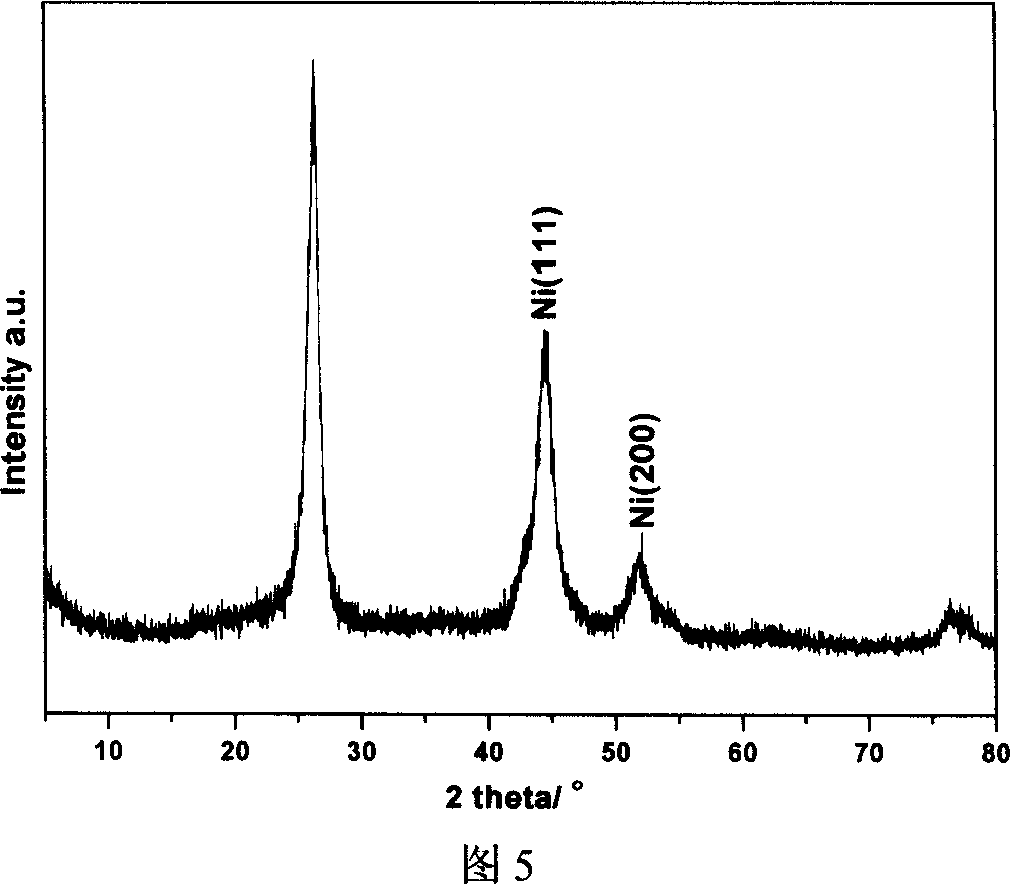
[0030] Weigh 0.212g Ni(AC) 2 Add 0.5g mixed acid-treated carbon nanofibers into 50mL ethylene glycol, ultrasonically disperse for 5 minutes, then raise the temperature to 160°C, add 0.9mL 0.2M Na 2 CO 3 The aqueous solution was kept at this temperature for 1 hour, then filtered, washed with water, and dried to obtain a catalyst precursor. The obtained precursor was placed in a tubular heating furnace, and the temperature was raised to 400°C at 3°C / min in a high-purity nitrogen atmosphere, kept for 4 hours, and then switched to nitrogen and hydrogen mixed gas for reduction for 1 hour to obtain carbon nanofiber-supported metal nickel catalyst. ICP results showed that the mass percent content of metallic nickel was 6.6%. The XRD calculation results show that the average particle size of metallic nickel particles is 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com