A composition for wound healing and use thereof
A composition and wound technology, applied in the field of accelerated wound healing, can solve the problems that diabetic foot ulcers are easily infected and cannot be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
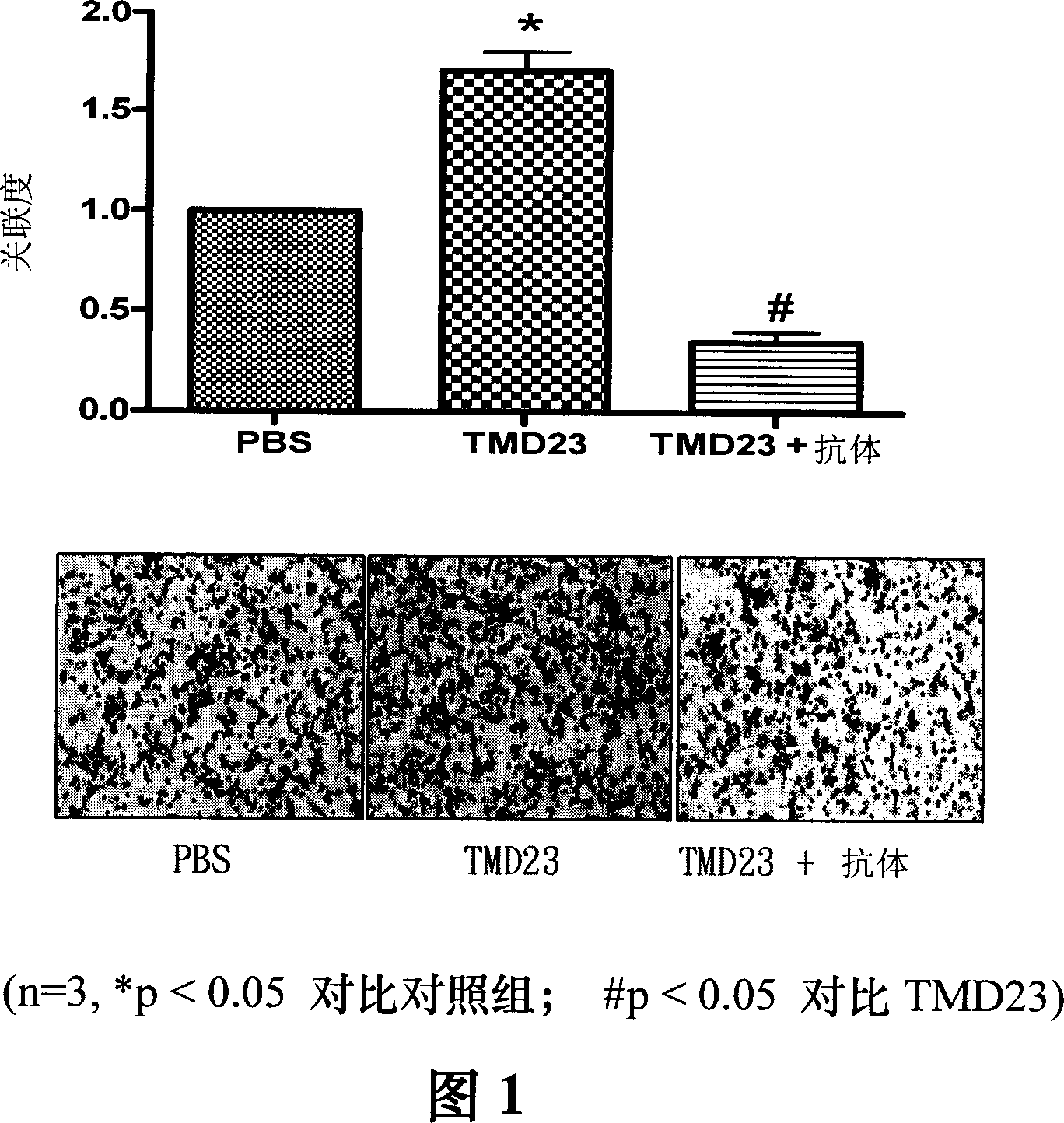
[0077] Example 1: Effect of TMD23 on migration of HaCaT epidermal cells
[0078] The effect of TMD23 on the migration of HaCaT cells was examined using a Boyden chamber with a 6.5 mm diameter polycarbonate filter membrane (8-μm pore size). Collagen type IV is coated on the surface of the lower filter membrane. Add TMD23 (100 ng / ml) or TMD23 (100 ng / ml) and anti-TM antibody (1 μg / ml) to DMEM in the lower well. Each upper well was added with 1 × 10 4 cell suspension (50 μL). Eight hours later, fixed with methanol and stained with 10% GIEMSA, the number of cells migrated through the membrane was counted under a microscope. As shown in Figure 1, TMD23 significantly induced the chemotactic migration of HaCaT cells.
Embodiment 2
[0079] Example 2: Materials
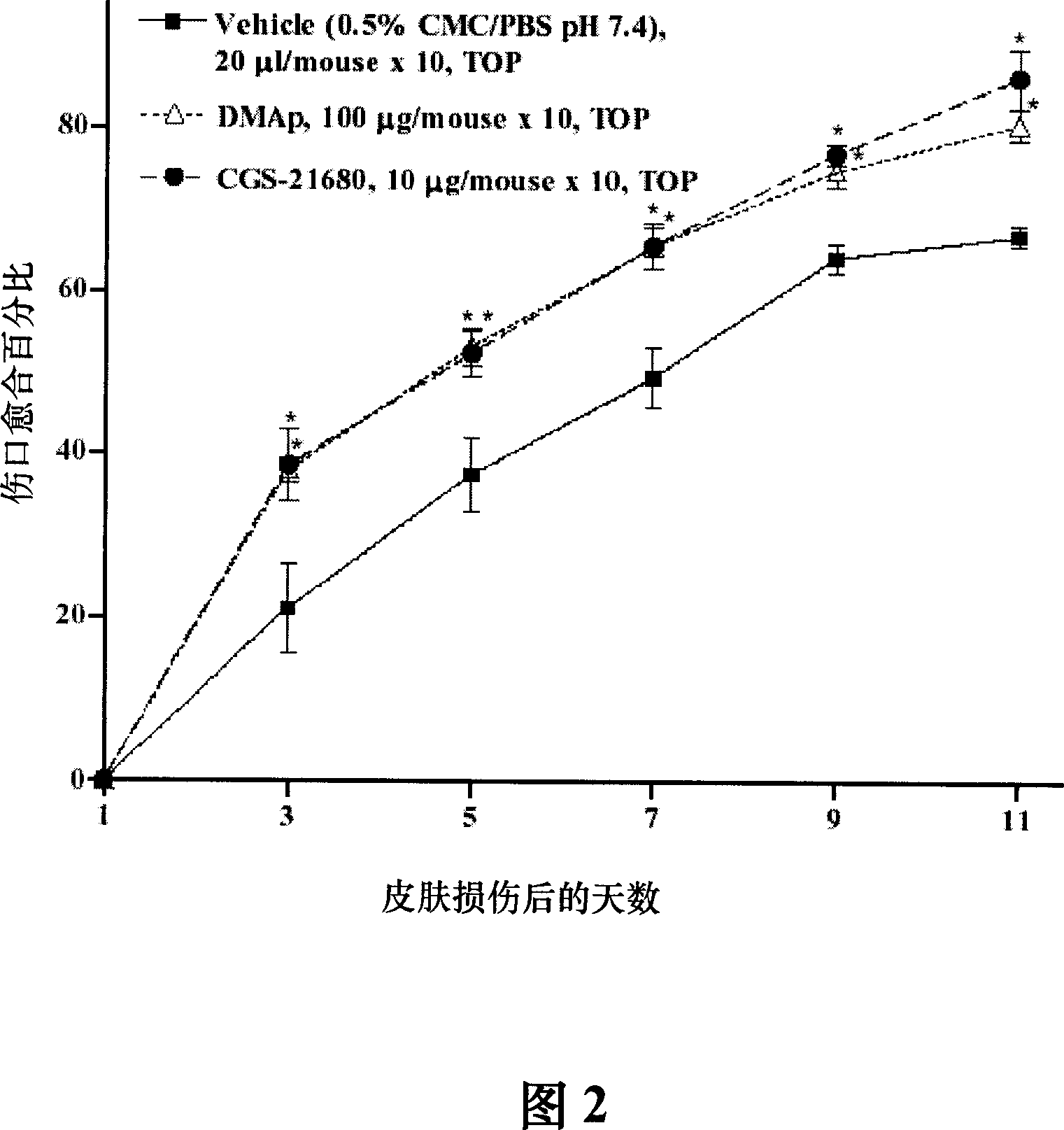
[0080] DMAp is 100 μg recombinant TMD23 purified protein (SEQ ID NO. 1 ) dissolved in 20 μl 0.5% CMC / PBS solution. 0.5% CMC (carboxymethylcellulose) / PBS (phosphate buffered saline) (pH 7.4) was used as a carrier for DMAp and CGS-21680 solutions. CGS-21680 hydrochloride (2-p-[2-carboxymethyl]anilino-5'-N-ethylcarboxamidoadenosine), a G protein activator, was purchased from Sigma-Aldrich.
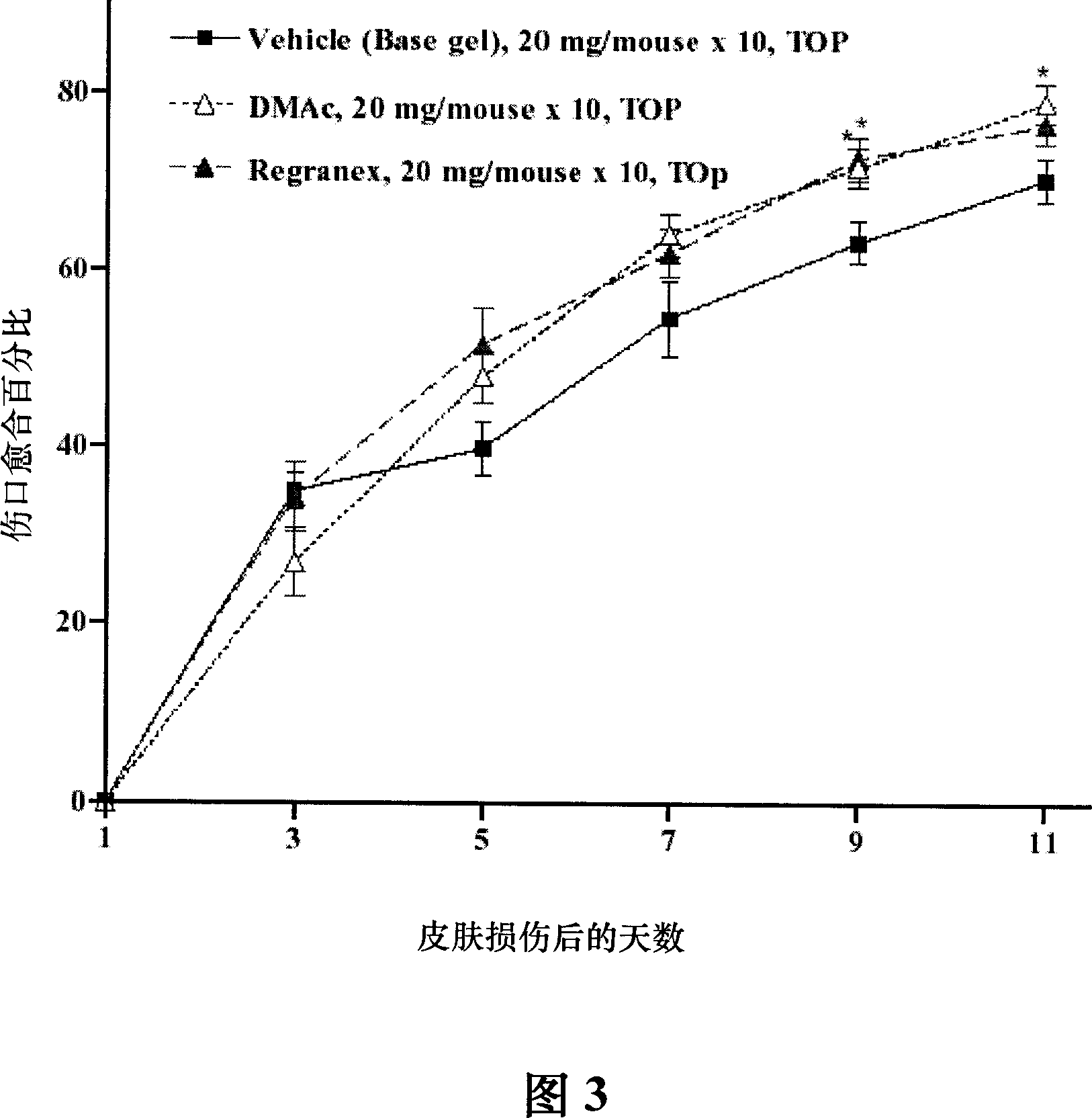
[0081] DMAc gel is 100 μg recombinant TMD23 purified protein (SEQ ID NO. 1 ) dissolved in 20 mg base gel. The basic gel above was used as a negative control for DMAc gel and Regranex gel. The above basic gel is composed of sodium carmellose, sodium chloride, sodium acetate trihydrate, glacial acetic acid, methylparaben, propylparaben, m-cresol 1-lysine hydrochloride, benzyl alcohol , methylchloroisothiazoline (methylchloroisothiazoline), methylisothiazoline (methylisothiazoline), ammonium acryloyldimethyltaurate / VP copolymer (ammoniumacryloyldimethyltaurate / ...
Embodiment 3
[0084] Example 3: Animals
[0085] CD-1 (Crl.) male mice weighing 24±2 g were provided by Taiwan BioLasco (licensed by Charles River Laboratories). A space of 29 x 18 x 13 cm was partitioned for 10 mice. All mice were kept in MDS Medical Service Company (Taiwan laboratory is preferred) for at least one week, maintaining a 12-hour light-dark cycle and controlled temperature (22°C-24°C) and humidity (50%-60%) environment. Standard laboratory mouse chow [MF-18 (Oriental Yeast Co., Ltd., Japan)] and RO water were freely available. Work including housing construction, experiments, and animal handling were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington D.C., 1996).
[0086] Non-insulin-dependent diabetic (NIDDM) male mice (C57BLKS / J-m+ / +Lepr db) weighing 50±5 g (9 weeks old) provided by the Institute of Animal Reproduction (IAR, Japan) were used. These mice all showed hyperinsulinemia, hyperglycemia and is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com