Poly N-isopropyl-acrylic-amide-poly amino-acid two-block copolymer and preparing method
A technology of isopropylacrylamide and block copolymer, which is applied in the field of poly-N-isopropylacrylamide-polyamino acid two-block copolymer and its preparation, can solve the problem of few reports on polyamino acid copolymerization and no copolymerization. things, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
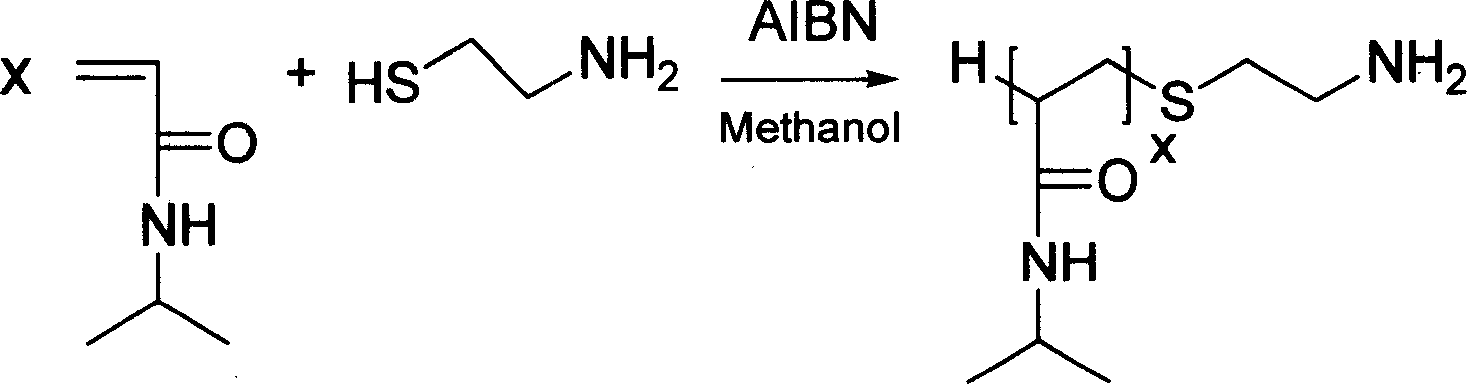
[0032] Example 1: Preparation of poly-N-isopropylacrylamide with amino terminal at one end
[0033] Weigh 3 parts of 0.2mol of N-isopropylacrylamide and 0.002mol of azobisisobutyronitrile, add them to 3 reaction ampoules respectively, then add 0.003, 0.006, 0.012 and 0.02mol of Mercaptoethylamine. After dissolving with 80ml of methanol respectively, the gas in the bottle was removed through 3 freeze-vacuum-melt cycles, and the ampoule was sealed. The solution was stirred at 50, 60, 60 and 80°C for 24 hours, and the product was settled with excess ether, filtered, washed, and vacuum-dried to obtain poly-N-isopropylacrylamide homopolymers of different molecular weights with one end being an amino terminal. The molecular weight of each product was calculated by NMR. The obtained polymer results are shown in Table 1.
[0034] Table I:
[0035] Numbering
[0036] In the above table, A / I / T refers to the molar ratio of N-isopropylacrylamide monomer / azobisisobutyronitri...
Embodiment 2
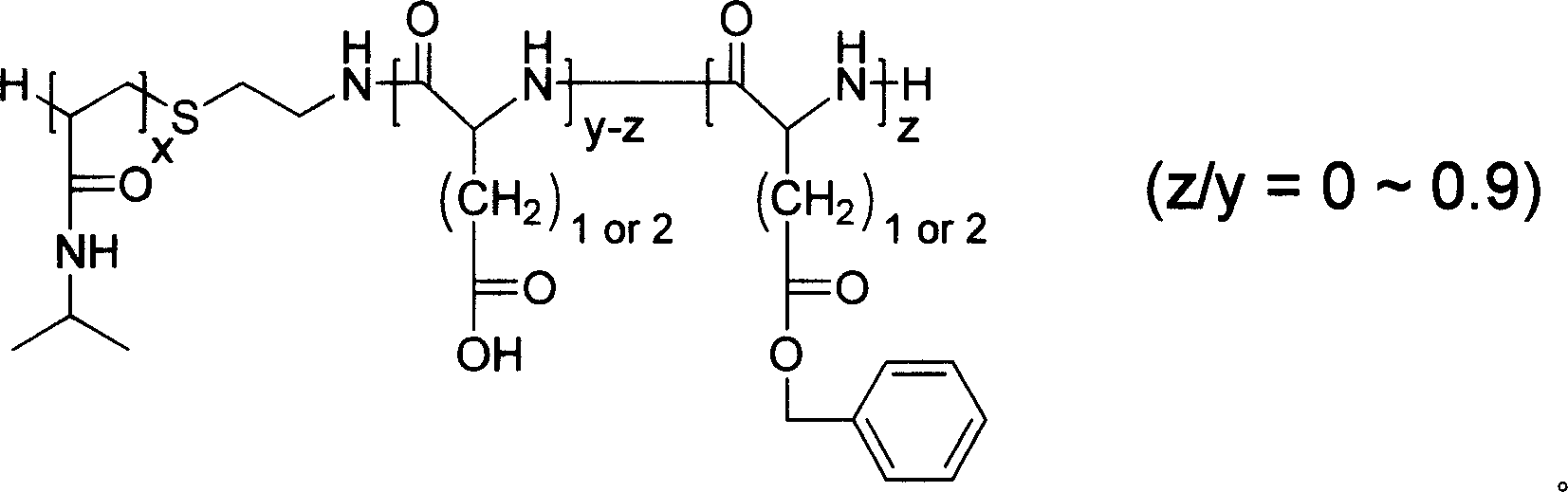
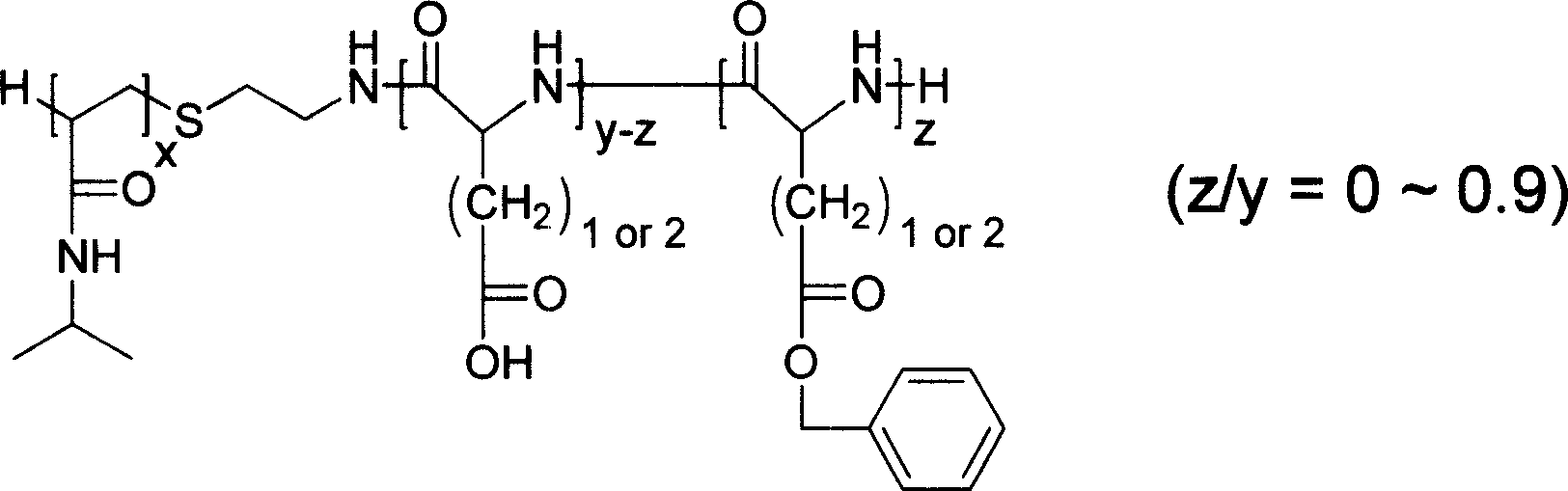
[0037] Example 2: Preparation of poly-N-isopropylacrylamide-poly-γ-benzyl-L-glutamate diblock copolymer
[0038] Respectively, 10 g of poly-isopropylacrylamide (PNIPAM) and measured gamma-benzyl-L-glutamic acid ester-N-carboxylic acid anhydride (BLG-NCA) were used 30 times (volume and weight) respectively. ratio, ml / g) of dimethyl sulfoxide dissolved. Add the BLG-NCA / DMSO solution to the rapidly stirred poly-N-isopropylacrylamide / DMSO solution at room temperature. After the solution was stirred at 25° C. for 72 hours, it was dialyzed in ethanol to remove impurities and unreacted poly-N-isopropylacrylamide homopolymer. The colloidal solid obtained was dissolved in tetrahydrofuran, settled with ether, and vacuum-dried to obtain a poly-N-isopropylacrylamide-poly-γ-benzyl-glutamate (PNIPAM-PBLG) diblock copolymer. The results are shown in Table 2:
[0039] Table II:
[0040] Numbering
[0041] M in the above table n (PNIPAM) refers to the number-average molecular w...
Embodiment 3
[0042] Example 3: Preparation of poly-N-isopropylacrylamide-poly-β-benzyl-L-aspartate diblock copolymer
[0043] 2 g of poly-isopropylacrylamide (M n =3700, DP=32) and 4g β-benzyl-L-aspartic acid (BLA-NCA) were dissolved with 60ml and 120ml of dimethyl sulfoxide respectively, and other steps and conditions were the same as in Example 2 to obtain polyN - Isopropylacrylamide-poly-β-benzyl-aspartate (PNIPAM-PBLA) diblock copolymer. Productive rate: 93.6%; DP (PBLA)=28 (number average molecular weight 5700), DP (PBLA) by 1 H NMR measurement obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com