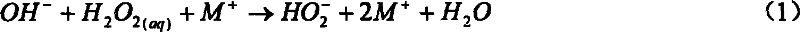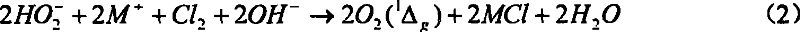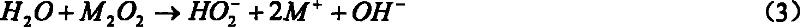Method for confecting chemical fuel of oxygen-iodine chemical laser
A technology of chemical fuel and oxygen-iodine chemistry, which is applied to laser components and active dielectric materials, can solve the problems of cumbersome, complicated, unsafe and slow process of oxygen-iodine chemical laser operation, and achieve easy acquisition, easy storage and transportation, and preparation Safe and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the chemical fuel prepared by lithium peroxide and water
[0037] At room temperature and normal pressure, add 2g of off-white lithium peroxide powder into 60ml of ice distilled water, stir and mix to completely dissolve lithium peroxide in ice water. Lithium peroxide needs to be stirred for a certain period of time to completely dissolve in ice heavy water. The dissolution process is relatively mild, with little heat release and less oxygen evolution. Then add this solution in a 100ml three-necked flask, vacuumize, keep the reactor pressure below 100torr, magnetically stir, then feed chlorine gas through a glass gas bubbler with evenly distributed small holes at the bottom to react, and the reactor pressure remains At 150~300torr. Red light can be clearly observed in the reaction. Point the optical fiber head coupled with the OMA-V spectrometer and the CCD spectrometer directly to the three-necked flask to monitor the radiation signal of singlet oxygen....
Embodiment 2
[0038] Embodiment 2: the chemical fuel prepared by lithium peroxide and heavy water
[0039] The preparation process and phenomenon of chemical fuel, the experimental process of reacting with chlorine and the test method of singlet oxygen are all the same as in Example 1, except that 60ml of water is replaced by 60ml of heavy water. The red light can be clearly observed in the reaction, and the spectrometer can easily monitor the near-infrared 1266nm unimolecular radiation signal of singlet oxygen and the bimolecular synergistic radiation signal of 6340nm or 703nm in the visible light region, except for these three radiations In addition to the spectral peak, the O near 762nm was also measured 2 ( 1 ∑ g + ) radiation spectrum peak.
Embodiment 3
[0040] Embodiment 3: the chemical fuel prepared by sodium peroxide and water
[0041]At room temperature and normal pressure, add 6g of yellow sodium peroxide powder into 60ml of ice-water mixture, stir and mix to completely dissolve the sodium peroxide in the ice-water, and the sodium peroxide dissolves rapidly when added to water, releasing a large amount of heat and energy Oxygen evolves. Then add this solution in a 100ml three-necked flask, vacuumize, keep the reactor pressure below 100torr, magnetically stir, then feed chlorine gas through a glass gas bubbler with evenly distributed small holes at the bottom to react, and the reactor pressure remains At 150~300torr. Red light can be clearly observed in the reaction. Point the optical fiber head coupled with the OMA-V spectrometer and the CCD spectrometer directly to the three-necked flask to monitor the radiation signal of singlet oxygen. The spectrometer can easily monitor the near-infrared 1266nm single molecule radia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com