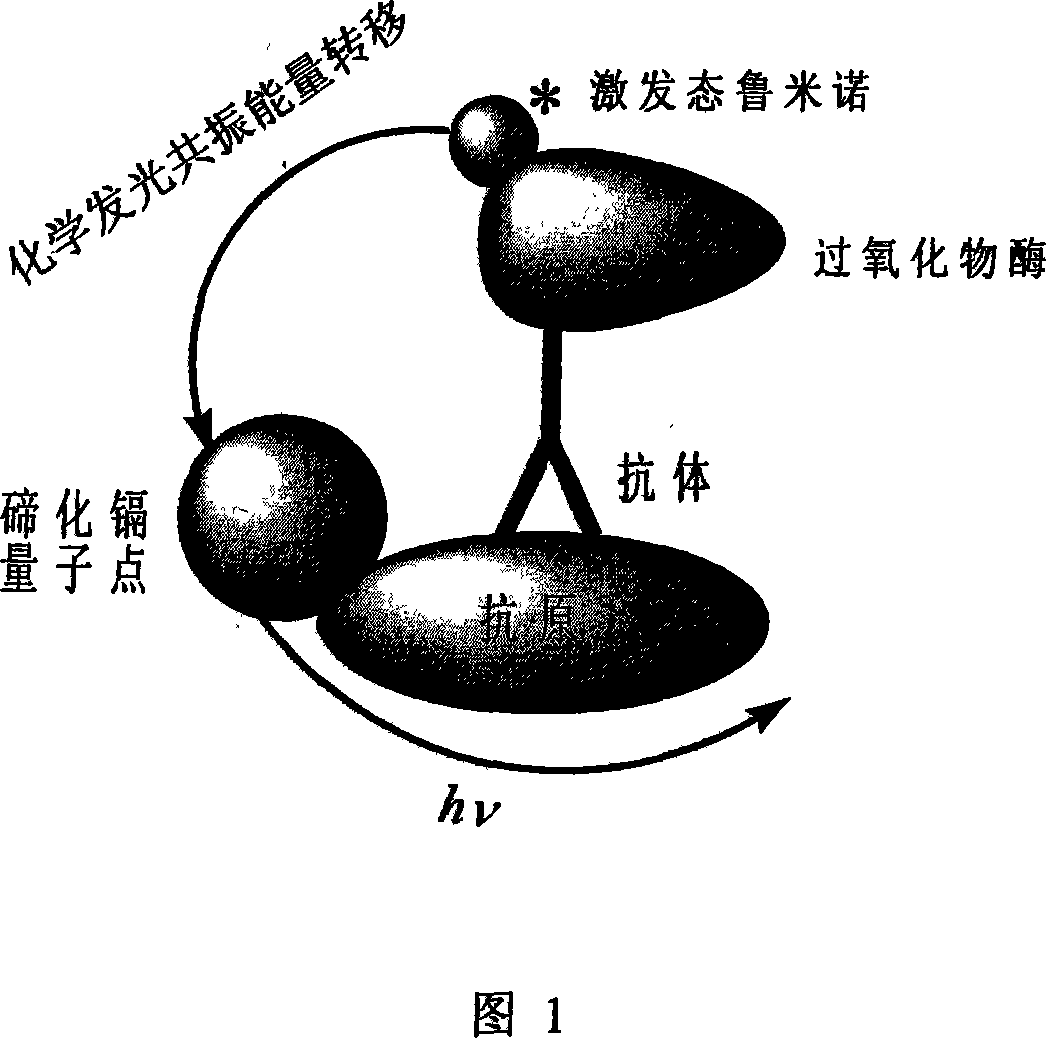
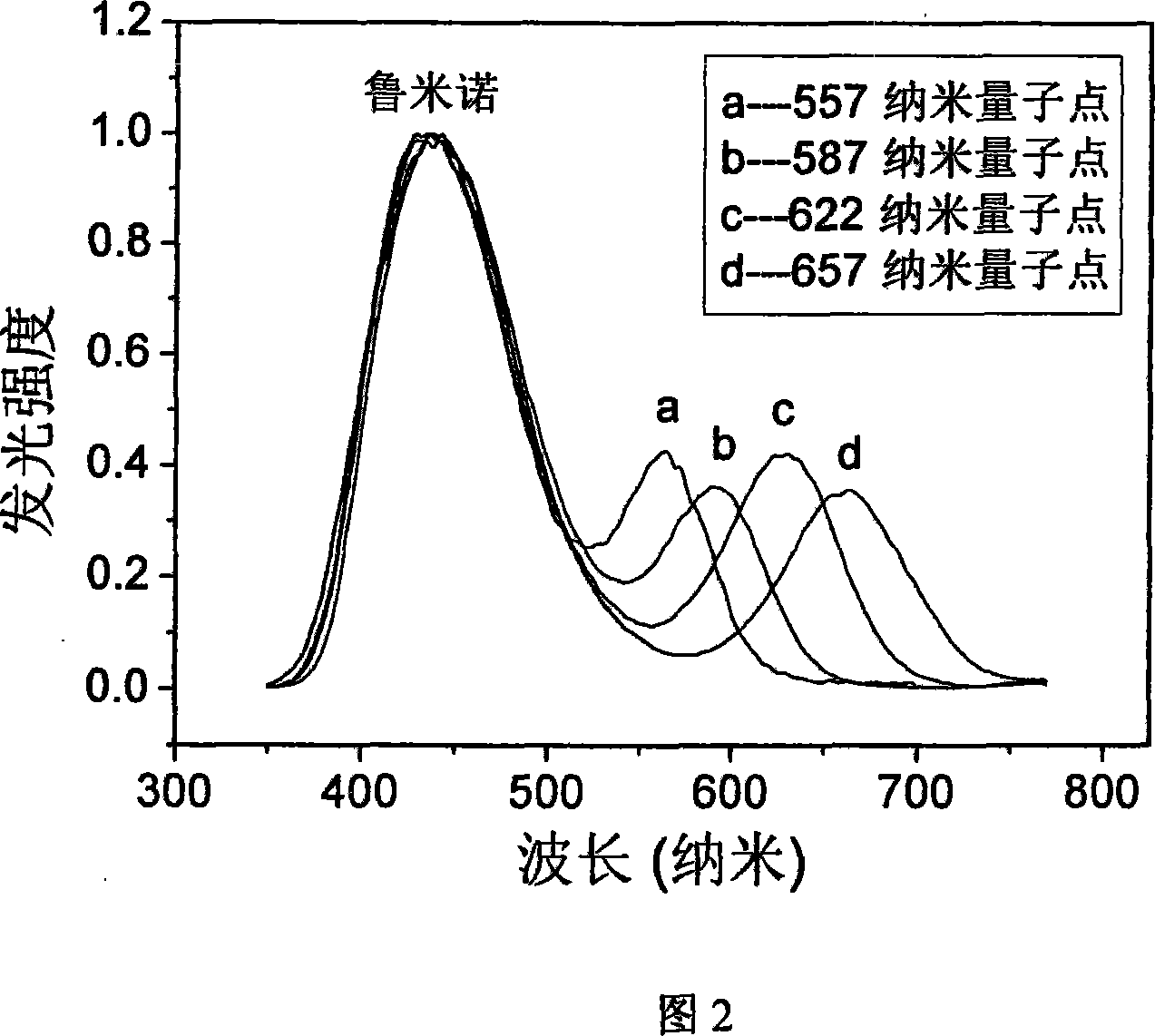
Homogeneous immune analysis nano device construction method
A nano-device and immune analysis technology, which is applied in the direction of chemical reaction analysis of materials, material inspection products, chemiluminescence/bioluminescence, etc., can solve the complex structure of the fluorescence resonance energy transfer analysis system, expensive instruments, and increased background Interference and other problems, to achieve the effect of wide luminous range, easy operation, good water solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) sodium telluride hydride preparation:
[0032] Put 80 mg of tellurium powder, 80 mg of sodium borohydride and 2 ml of water in a 10 ml small flask and react at room temperature for 8 hours to prepare a sodium telluride hydride solution.
[0033] (2) Synthesis of water-soluble cadmium telluride quantum dots:
[0034]Mix 0.00125 mol / L cadmium chloride and 0.003 mol / L mercaptopropionic acid to form a solution, adjust the pH value to 8.0-9.0 with sodium hydroxide solution, pass nitrogen gas to remove oxygen for 30 minutes, stir vigorously and in nitrogen gas Under protection, inject sodium hydride solution to form cadmium telluride monomer solution. The molar ratio of added divalent cadmium ions: sodium hydride telluride: mercaptopropionic acid is 2:1:4.8. Put the obtained precursor solution into a microwave digestion tank and heat it by microwave radiation. The reaction time is controlled for 0.5-2 hours to obtain the water-soluble cadmium telluride quantum dot with...
Embodiment 2
[0042] (1) The preparation of sodium telluride hydride is the same as in Example 1.
[0043] (2) The synthesis of water-soluble cadmium telluride quantum dots is the same as in Example 1.
[0044] (3) Preparation of cadmium telluride quantum dot-cancer antigen 125 (CA125) biomarker
[0045] Mix 0.3 mg / ml cadmium telluride quantum dots, 0.6 mg / ml EDC and 0.3 mg / ml CA125 in 0.01 mol / L phosphate buffer solution with a pH value of 7.0, and react the mixed solution at room temperature in the dark for 2 -4 hours. Quantum dot biomarkers were purified by ultrafiltration. Finally, store it in the refrigerator at 4 degrees Celsius for later use.
[0046] (4) Preparation of CA125 antibody-peroxidase marker
[0047] Get 0.2 mg / ml peroxidase solution, 0.2 mg / ml EDC solution and 0.3 mg / ml N-hydroxysuccinimide in 0.01 mol / liter phosphate buffer solution with a pH value of 7.0 for about 15 minutes, The reaction solution was purified by ultrafiltration. Then add the CA125 antibody soluti...
Embodiment 3
[0051] (1) The preparation of sodium telluride hydride is the same as in Example 1.
[0052] (2) The synthesis of water-soluble cadmium telluride quantum dots is the same as in Example 1.
[0053] (3) Preparation of cadmium telluride quantum dot-cancer antigen 19-9 (CA19-9) biomarker
[0054] Mix 0.05 mg / ml cadmium telluride quantum dots, 0.1 mg / ml EDC and 0.05 mg / ml CA19-9 in 0.01 mol / L phosphate buffer solution with a pH value of 7.0, and store the mixed solution at room temperature in the dark React for 2-4 hours. Quantum dot biomarkers were purified by ultrafiltration. Finally, store it in the refrigerator at 4 degrees Celsius for later use.
[0055] (4) Preparation of CA19-9 antibody-peroxidase marker
[0056] Containing 0.04 mg / ml peroxidase solution, 0.04 mg / ml EDC solution and 0.06 mg / ml N-hydroxysuccinimide in 0.01 mol / L, pH value of 7.0 phosphate buffer for about After 15 minutes, the reaction solution was purified by ultrafiltration. Then, 0.1 mg / ml CA19-9 ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com