Nucleoside diphosphokinase A oxidation-reduction isomer
A technology of nucleoside diphosphate kinase and isomer, applied in the field of genetic engineering, can solve the problems of loss, loss of NDPK activity, loss of protein phosphotransferase activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Construction of mutant Nm23-H1 gene by PCR method
[0043] 1. Purpose
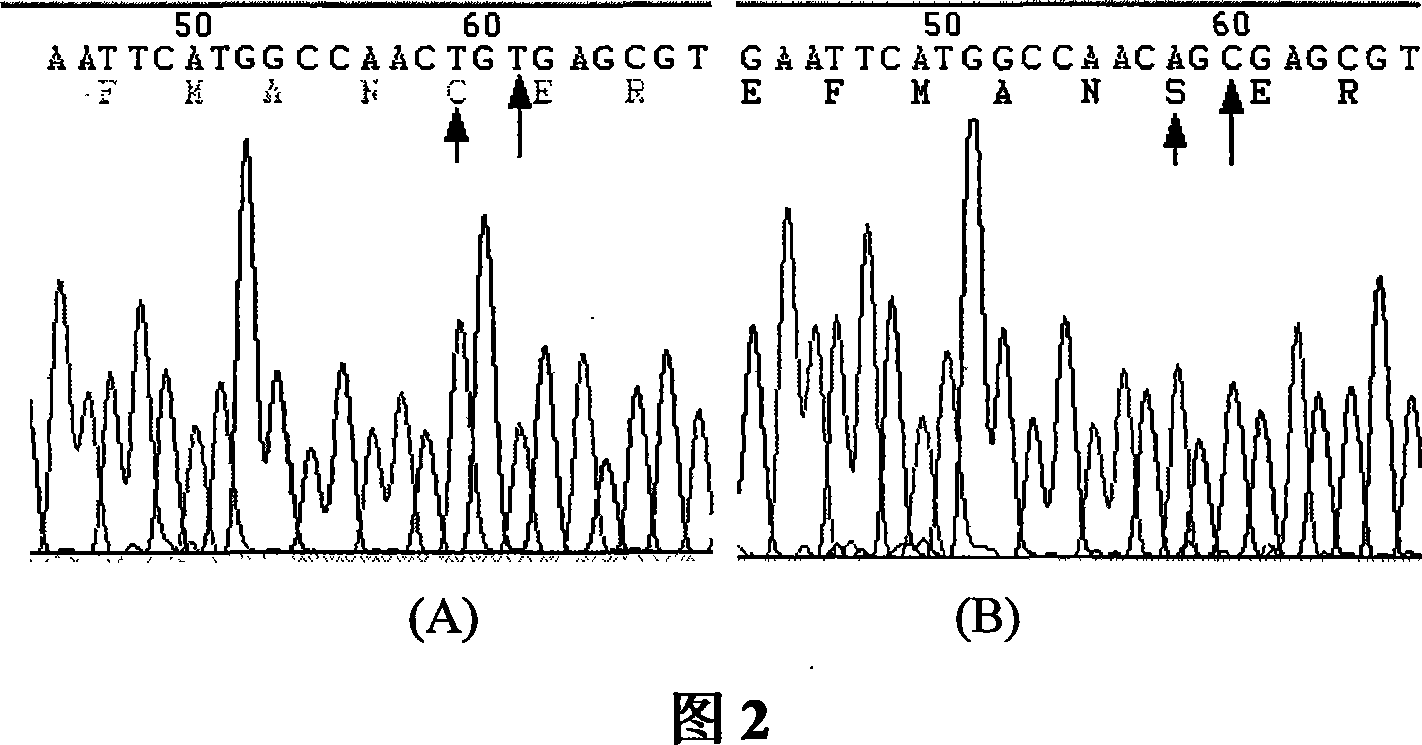
[0044] In the previous work, we found (Xiong Sheng, Qian Chuiwen, Wang Yifei, et al. Research on the Isomerization and Mechanism of Nucleoside Diphosphate Kinase A. Chinese Journal of Biochemistry and Molecular Biology. 2005), recombinant human NDPK-A can be Isomerization occurs due to oxidation and reduction, and some physicochemical properties and enzyme activities of isomers are significantly different. These phenomena suggest that the mutation of key residues (Cys) related to redox isomerization of NDPK-A may improve its activity.
[0045] 2. Experimental materials
[0046] The expression plasmid pBV-Nm23H1 containing the wild-type NDPK-A gene was constructed by Hou Yu et al. and kept in our laboratory (Hou Yu et al. Expression of Nm23-H1 / NDPK-A gene in Escherichia coli and purification of its product. Chinese Journal of Biochemistry and Molecular Biology, 1998), E.coli DH5α was pu...
Embodiment 2
[0061] Embodiment 2: Construction of NDPK-A C4S engineering bacteria
[0062] 1. Purpose
[0063] Construction of genetically engineered bacteria capable of highly expressing NDPK-A C4S mutant protein.
[0064] 2. Experimental materials
[0065] Recombinant plasmid pUC-Nm23H1 C4S containing NDPK-A C4S gene (constructed by Example 1), E.coliDH5α was purchased from Promega Company, expression vector pBV220 (Zhang Zhiqing et al. containing P_RP_L promoter, prokaryotic high-efficiency expression vector, and its application virus Acta 1990).
[0066] 3. Experimental method
[0067] Preparation of exogenous gene fragments: E.coli DH[pUC-Nm23H1 C4S] single colony inoculates 5mL LB culture medium, cultivates overnight at 37°C and 220rpm, extracts plasmid DNA by mini-extraction method, double enzyme digestion with EcoR I and BamH I, enzyme digestion The product was subjected to agarose gel electrophoresis, and small fragments were recovered from the gel, and stored at -20°C for fut...
Embodiment 3
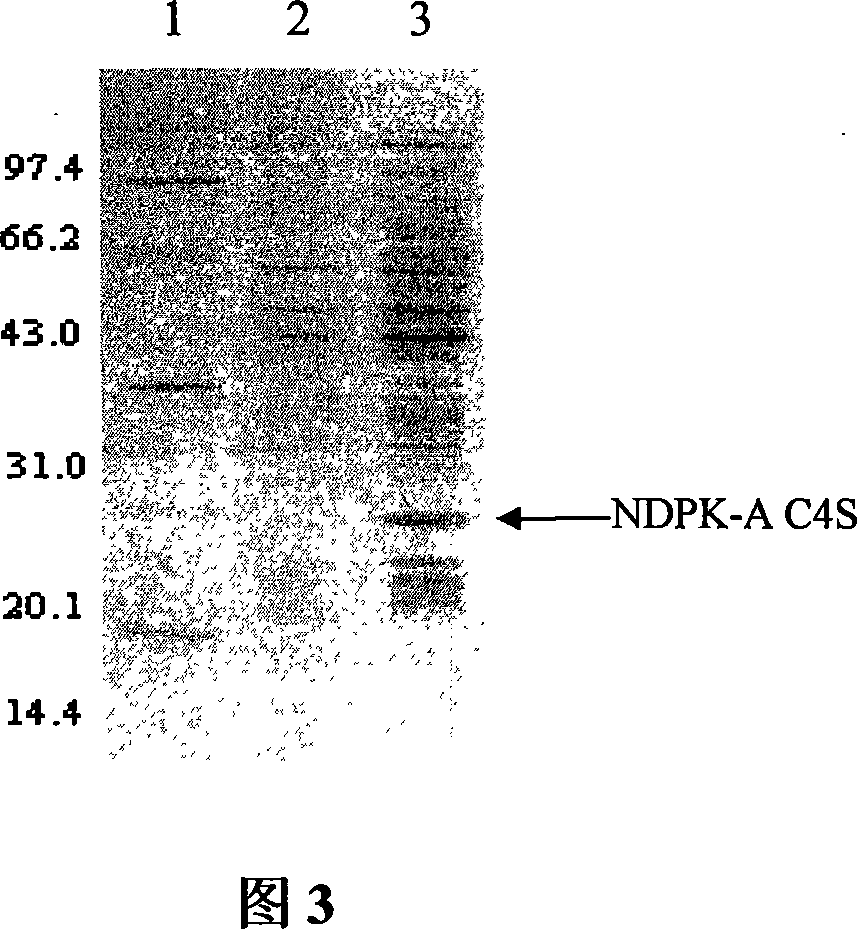
[0075] Example 3: Expression and purification of NDPK-A isoforms
[0076] 1. Purpose
[0077] Prepare NDPK-A C4S isoform protein to provide materials for subsequent experiments such as activity determination.
[0078] 2. Experimental materials
[0079] The engineering bacteria E.coli DH[pBV-Nm23-C4S] was preserved by the inventor's laboratory, and the construction method was listed in Example 2; the chromatography matrix DEAE-sepharose Fast Flow was purchased from Amersham Bioscience Company; Cibacron Blue3GA Sepharose CL-4B Purchased from Millipore Company;
[0080] Purification buffer and its formula: Buffer A: 20mmol / L Tris-HCl, 1mmol / L EDTA, 2mmol / LMgCl 2 , 1mmol / L DTT, pH7.5; Buffer B: Buffer A containing 0.05mol / L NaCl; Buffer C: BufferA containing 0.1mol / L NaCl; Buffer D: Buffer C containing 1.5mmol / L NaCl.
[0081] 3. Experimental method
[0082] 3.1 Shake flask fermentation and sample pretreatment
[0083] Inoculate 5mL LB medium with engineering bacteria DH[pBV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com