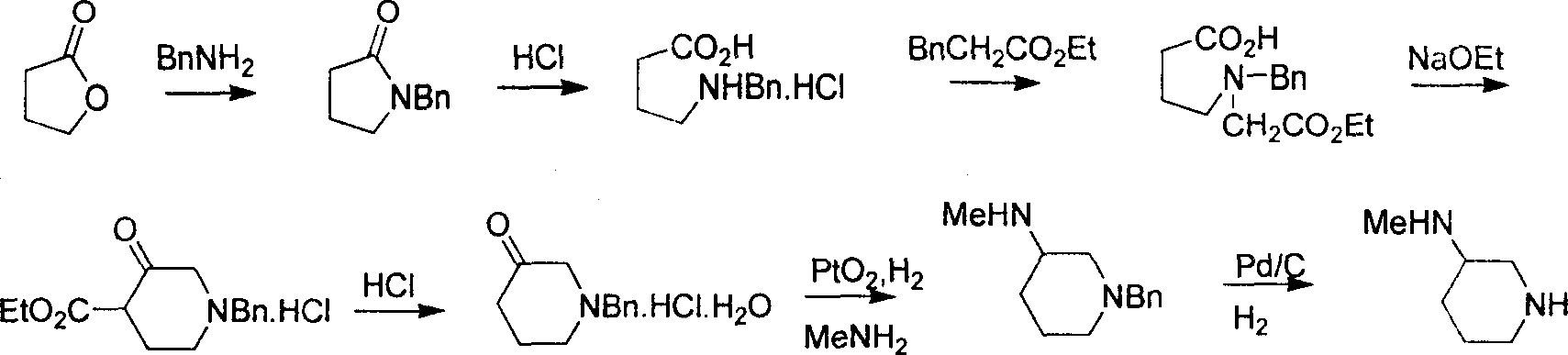
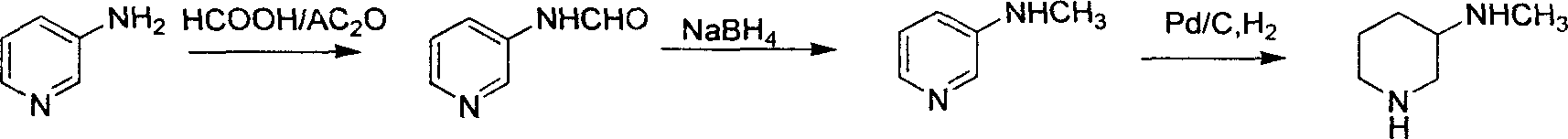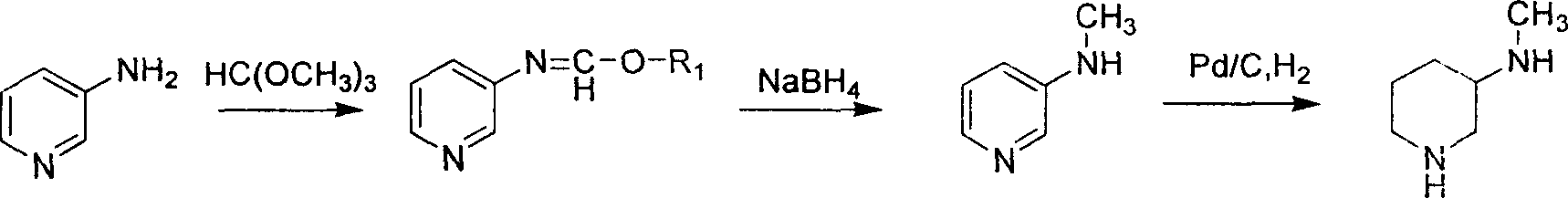Method for preparing methylamino hexahydropyridine
The technology of methylaminopiperidine and methylamino alcohol is applied in the field of preparation of 3-methylaminopiperidine, which can solve the problems of many by-products, difficult separation and purification, incomplete reaction and the like, achieves improved yield, simplified process operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1, the preparation of 3-methylaminopyridine (B)
[0044] (1) Add 250g (1.49mol) of 3-bromopyridine, 1200ml (12mol) of methylamine aqueous solution (30%) and 24.1g (0.1mol) of copper sulfate in a 2L autoclave, heat to 200°C, pressure 4MPa, constant temperature After 12 hours, cool and discharge, concentrate the reaction solution under reduced pressure, add 200ml of 40% sodium hydroxide solution, extract with 1000ml of dichloromethane in 3 times, dry over anhydrous sodium sulfate, and recover the solvent. The residue was distilled under reduced pressure, and the fraction at 133-134°C (15 mmHg) was collected to obtain 142 g of a colorless oily liquid, with a yield of 88.4%.
[0045] (2) Add 250g (1.49mol) of 3-bromopyridine and 1200ml (12mol) of methylamine solution (30%) into a 2L autoclave, heat to 200°C, pressure 4MPa, keep constant temperature for 12h, cool and discharge, concentrate under reduced pressure After the reaction solution, 200 ml of 40% sodium hydroxide so...
Embodiment 2
[0048] 2, Preparation of 3-methylaminopiperidine
[0049] In a 2L autoclave, add 108g (1mol) of 3-methylaminopyridine (B), add 40g of Pd / C catalyst, 1200ml of ethanol, carry out the reduction reaction at 120°C and 20MPa for 24h, filter out the catalyst after cooling, and recover the filtrate solvent. Concentrate under reduced pressure, add dichloromethane to dissolve, wash twice with water, separate the water phase, and dry the organic phase with anhydrous sodium sulfate. Atmospheric distillation removed dichloromethane, added petroleum ether, crystallized, filtered, and dried to obtain 8.5 g of white solid with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com