Tin-cobalt alloy negative electrode material for lithium ion cell and its preparing method
A lithium-ion battery, tin-cobalt alloy technology, applied in electrode manufacturing, battery electrodes, active material electrodes, etc., can solve the problem of large irreversible capacity, achieve the effect of not easy to agglomerate and surface oxidation, reduce irreversible capacity, and take less time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] First, a layer of tin is attached to the copper foil before the tin-cobalt alloy is electroplated. Then, tin-cobalt alloy is deposited. Cobalt has good ductility, which can inhibit the volume expansion generated when lithium-tin alloy is formed during charging and discharging and the pulverization of the electrode after multiple cycles. The electroplating process conditions were as follows.
[0028] Plating solution composition: 50g / L SnCl 2 2H 2 O, 15g / L CoCl 2 , 200g / K 4 P 2 o 7 ·3H 2 O, 10g / L citric acid 1, 10g / L glycine, 3g / L methionine, Ph=8~9.
[0029] Current density: 15mA / cm 2 .
[0030] Deposition time: 1h.
[0031] Post-processing: After the electrodeposited sheet was washed with deionized water, it was dried with a blower in cold air to obtain a tin-cobalt alloy negative electrode material, and the weight percentage of Co was 7.56%.
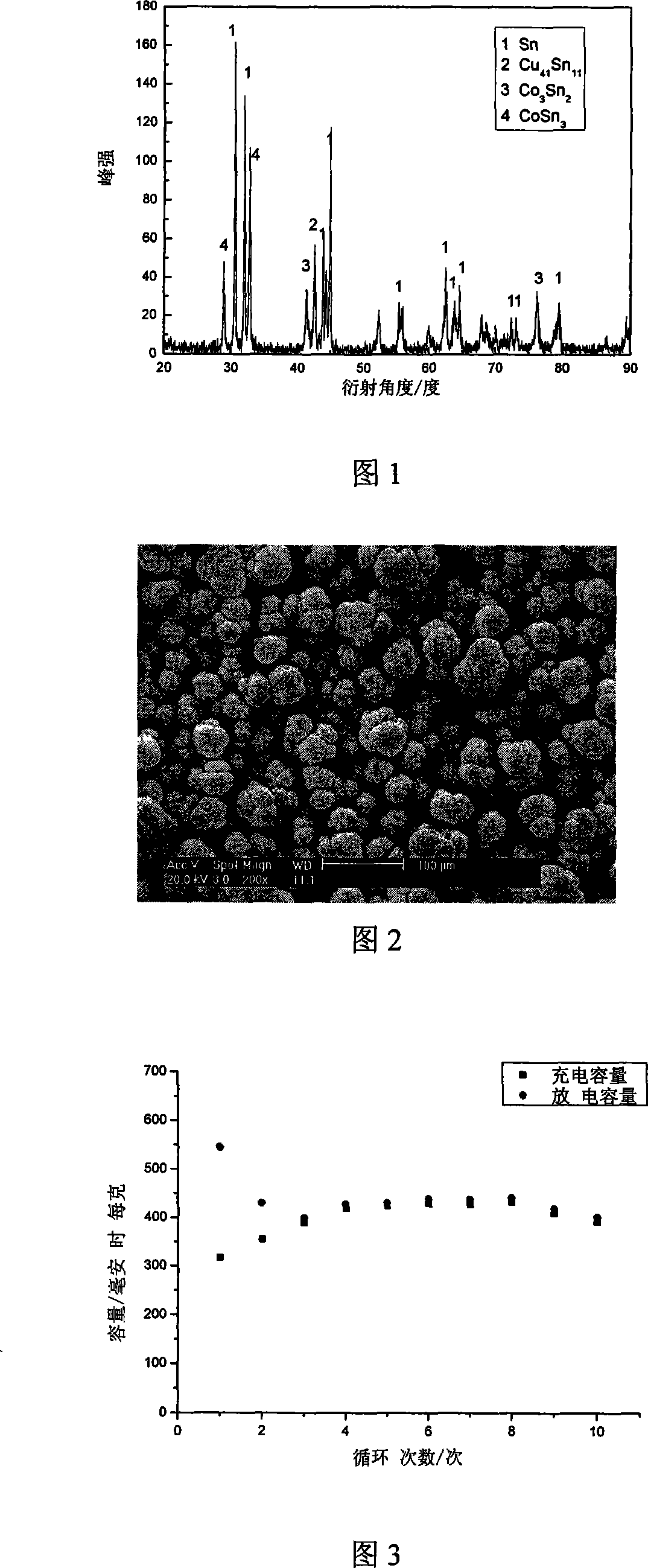
[0032] Fig. 1 is the XRD pattern of tin-cobalt alloy in embodiment 1, can determine that there is CoSn in the coati...
Embodiment 2
[0036] First, a layer of tin is attached to the copper foil before the tin-cobalt alloy is electroplated. Then, tin-cobalt alloy is deposited. Cobalt has good ductility, which can inhibit the volume expansion generated when lithium-tin alloy is formed during charging and discharging and the pulverization of the electrode after multiple cycles. The electroplating process conditions were as follows.
[0037] Plating solution composition: 30g / L SnCl 2 2H 2 O, 15g / L CoCl 2 , 200g / L K 4 P 2 o 7 ·3H 2 O, 10g / L citric acid 1, 10g / L glycine, 3g / L methionine, Ph=8~9.
[0038] Current density: 5mA / cm 2 .
[0039] Deposition time: 2.5h.
[0040] Post-processing: After the electrodeposited sheet is washed with deionized water, it is dried with a blower in cold air to obtain a tin-cobalt alloy negative electrode material, and the weight percentage of Co is 13.50%.
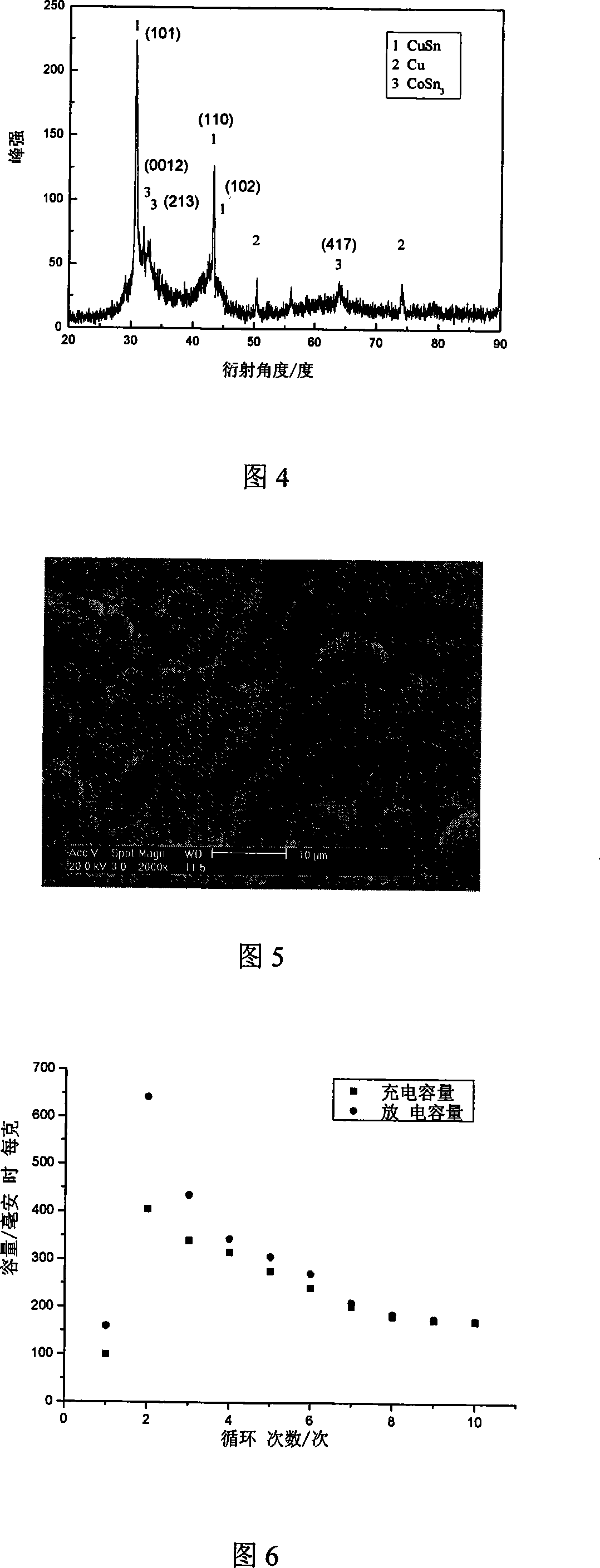
[0041] Fig. 4 is the XRD pattern of tin-cobalt alloy in embodiment 2, can confirm that CuSn and CoSn exist in the ...
Embodiment 3
[0045] First, a layer of tin is attached to the copper foil before the tin-cobalt alloy is electroplated. Then, tin-cobalt alloy is deposited. Cobalt has good ductility, which can inhibit the volume expansion generated when lithium-tin alloy is formed during charging and discharging and the pulverization of the electrode after multiple cycles. The electroplating process conditions were as follows.
[0046] Plating solution composition: 10g / L SnCl 2 2H 2 O, 15g / L CoCl 2 , 200g / L K 4 P 2 o 7 ·3H 2 O, 10g / L citric acid 1, 10g / L glycine, 3g / L methionine, Ph=8~9.
[0047] Current density: 20mA / cm 2 .
[0048] Deposition time: 0.5h.
[0049] Post-processing: After the electrodeposited sheet was washed with deionized water, it was dried with a blower in cold air to obtain a tin-cobalt alloy negative electrode material, and the weight percentage of Co was 22.63%.
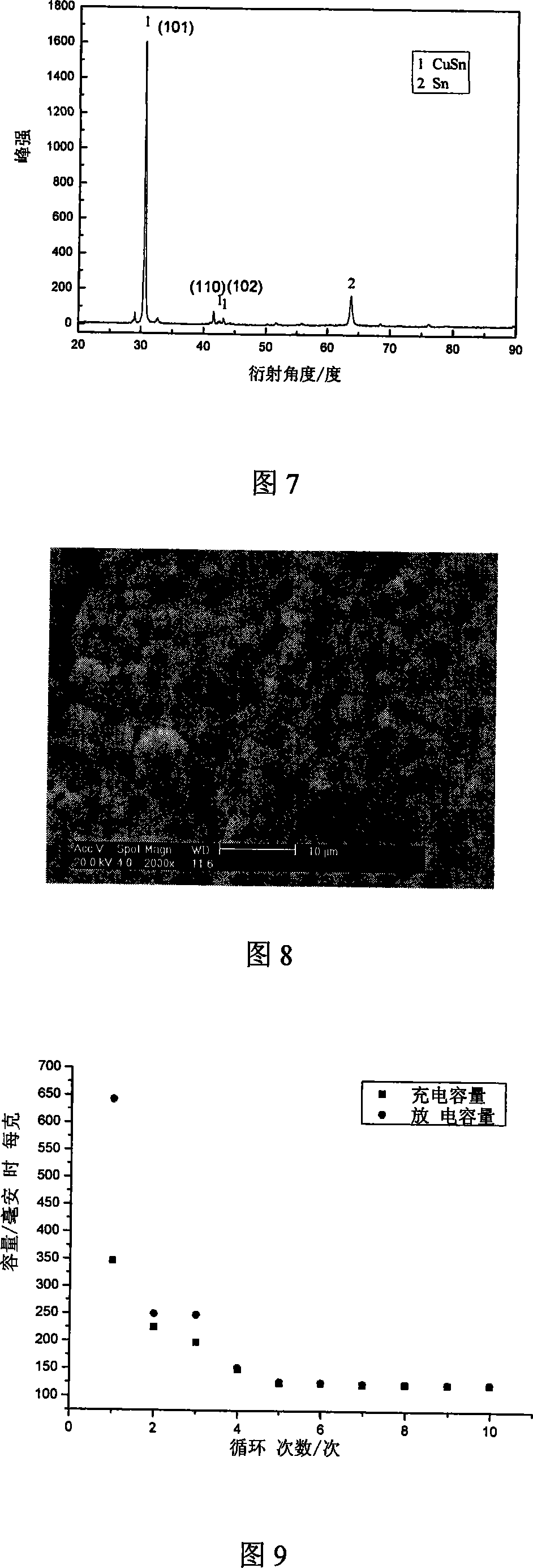
[0050] Figure 7 is the XRD pattern of the tin-cobalt alloy in Example 3, it can be confirmed that there is CuSn ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com