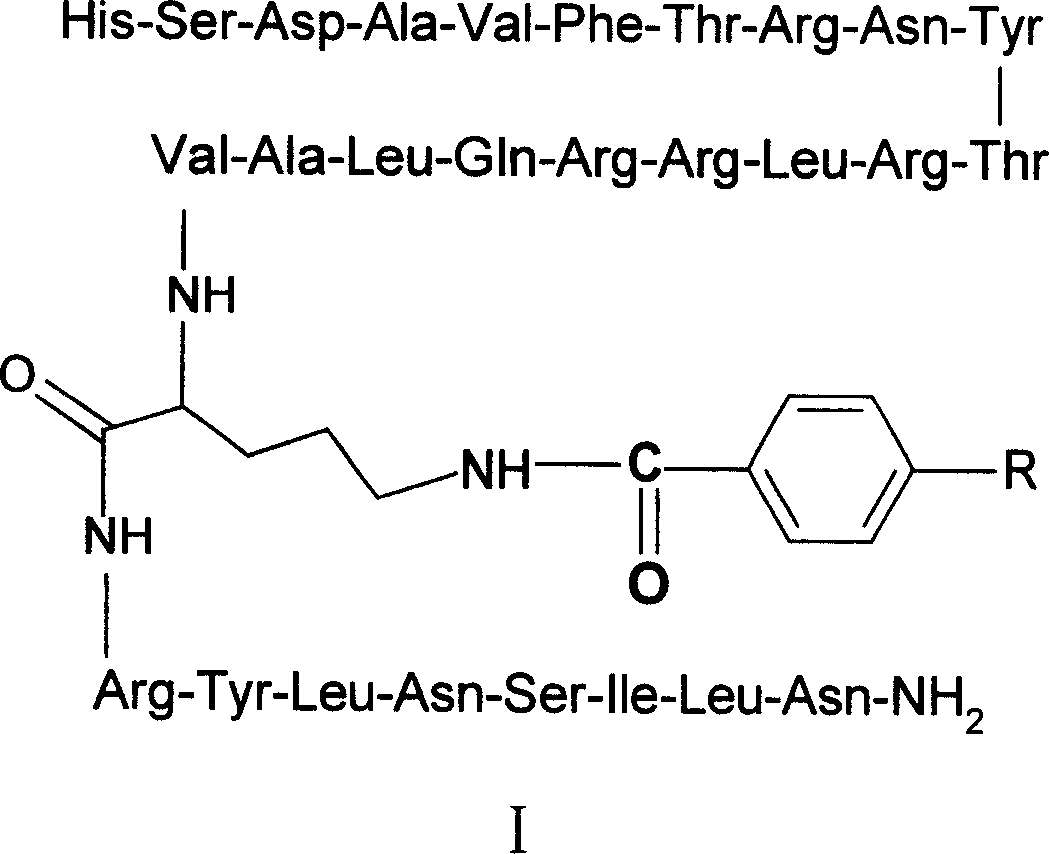
VIP analog and its radioactive marker and their prepn process
A technology of radioactive labeling and analogues, applied in the direction of radioactive carriers, animal/human peptides, peptide sources, etc., can solve unsatisfactory problems, affect stability in vivo, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0040] Embodiment 1 (R 8,15,21 , L 17 ) Synthesis of VIP
[0041] Manually synthesized by Fmoc / tBu solid-phase peptide synthesis method, the raw materials used are: Fmoc-LinkerAMResin, Fmoc-Gly, Fmoc-Leu, Fmoc-Lys(Boc), Fmoc-Thr(tBu), Fmoc-Glu(OtBu), Fmoc -His(trt), Fmoc-Ser(tBu), Fmoc-Asp(OtBu), Fmoc-Ala, Fmoc-Val, Fmoc-Phe, Fmoc-Arg(pbf), Fmoc-Asn(trt), Fmoc-Tyr( tBu), Fmoc-Gln (trt), Fmoc-Ile, Fmoc-Asn (trt), all purchased from Bachem Company, [2-(1 H-benzotriazol-1-yl)-1,1,3, 3-Tetramethyluronium hexafluorophosphate (HBTU) was purchased from Richelieu Biotechnoligies, 1-hydroxybenzotriazole (HOBT) was purchased from Sigma, and diisopropylethylamine (DIPEA) was purchased from Aldrich.
[0042] Fmoc-Linker AM Resin removes Fmoc protecting group with DMF containing 20% piperidine, washes 9 times with DMF, adds HBTU (1.2 equivalents) to the obtained resin, HOBt (3.6 equivalents) reacts at 0 ℃ for 30 minutes, then Use DIPEA (4.8 equivalents) to couple 1 equivalent of FMOC...
Embodiment 2
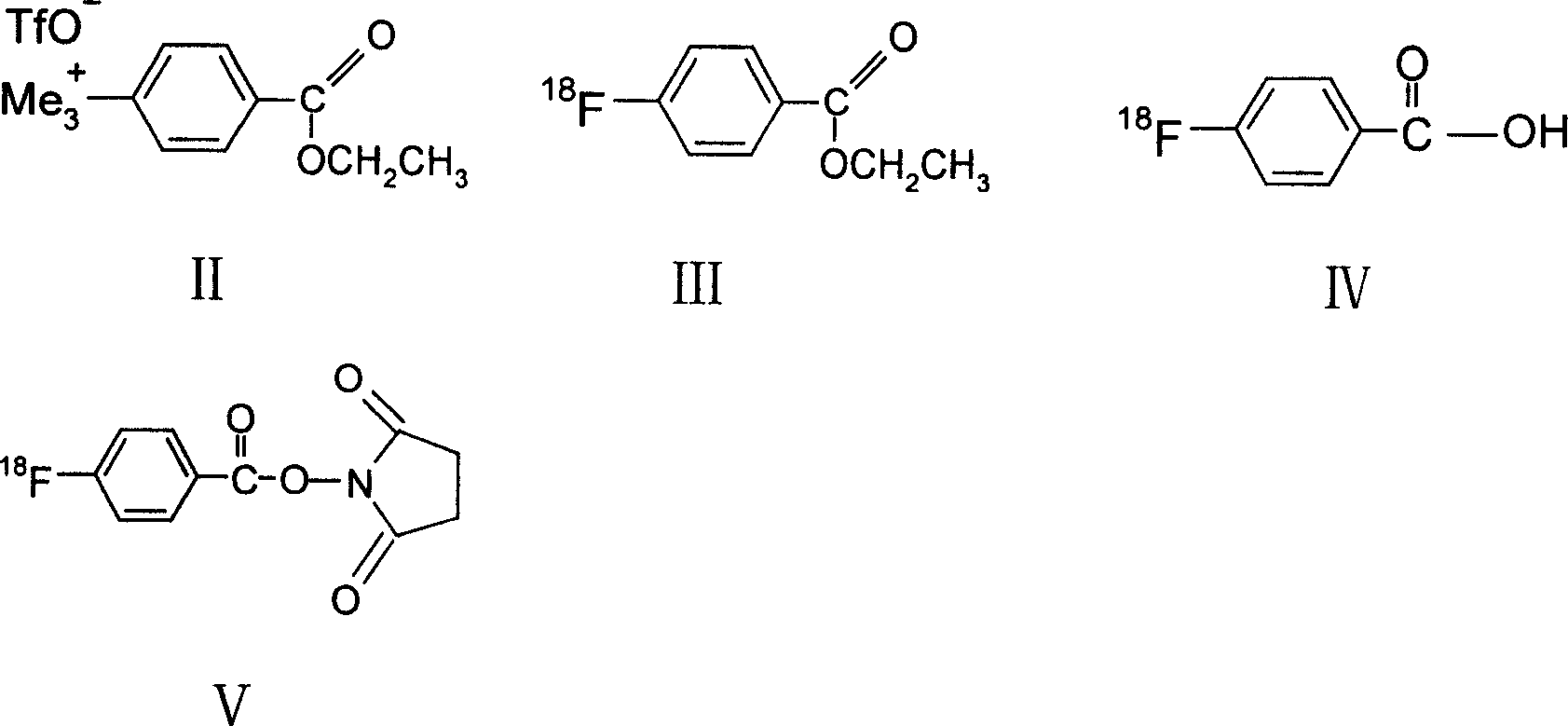
[0043] The synthesis of embodiment 2 compound 1
[0044] first step, 18 f - Activation, 100μL approximately 50mCi 18 f - Oxygen-enriched aqueous solution was added to the 2.2.2 and 1 mg of potassium carbonate in a conical reaction flask, heated in an oil bath at 90°C, fed nitrogen continuously, and dried the water. Then add 500 μL of acetonitrile, ventilate and blow dry; this process is repeated three times to ensure that the reaction system is completely anhydrous.
[0045] In the second step, 0.2 ml of anhydrous acetonitrile solution of 10 mg of formula II compound ethyl-4-trimethylamine benzoate-trifluorosulfonate was added to the dried 18 f - In the reaction bottle, carry out in 100 ℃ oil bath 18 f - Nucleophilic substitution reaction 10min obtains formula III compound 4-[ 18 F] ethyl fluorobenzoate. The product was characterized by radioactive thin-layer chromatography. The instrument used Bioscan system AR-2000 system (Bioscan Company, USA), the software was Wi...
Embodiment 3
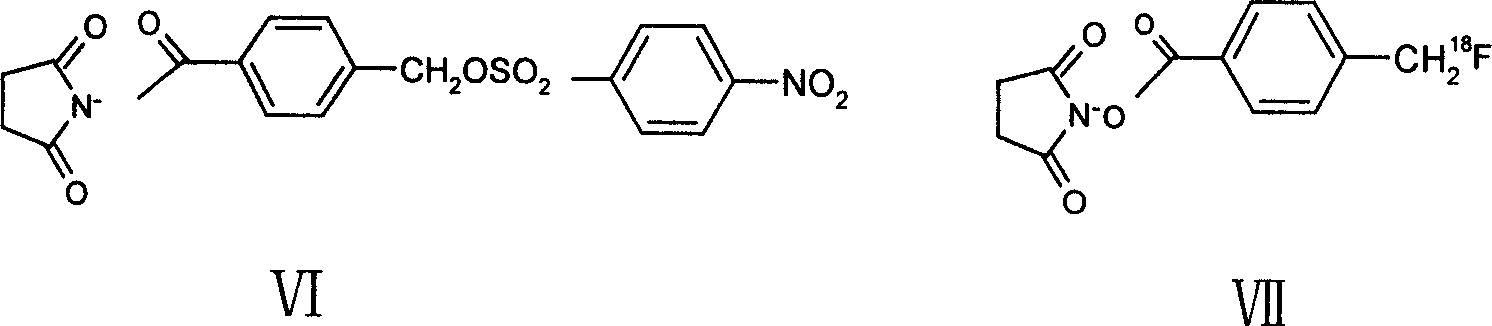
[0050] The synthesis of embodiment 3 compound 2
[0051] first step: 18 f - The activation, with the first step of embodiment 2.
[0052] The second step: anhydrous acetonitrile (300 μL) solution containing 4 mg of formula VI compound N-succinimide 4-[(4-nitrobenzenesulfonyl)oxymethyl]benzoate was added to the reaction flask, Sealed and reacted in an oil bath at 80°C for 10 minutes to obtain the compound of formula VII N-succinimide-4-[ 18 F] Fluoromethylbenzoate. The product is characterized by the radioactive thin-layer chromatograph used in the second step of Example 2, and R f=0.94, the labeling rate was 81.6%.
[0053] The reaction mixture was separated and purified using a Sep-Pak silica gel column (Waters), rinsed with 2 ml of dichloromethane / n-hexane (1:1, V / V), and then eluted with 2 ml of dichloromethane to obtain about 15 mCi of the product. The radiochemical purity was greater than 98%, and the radiochemical yield was 30% (uncorrected for decay).
[0054] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com