Making method of lithium iron phosphate compound anode material of lithium battery
A technology of lithium iron phosphate and composite materials, which is applied in the direction of electrode manufacturing, battery electrodes, phosphorus compounds, etc., can solve the problems of unfavorable industrial production, inability to mix materials, poor product performance, etc., and achieve high utilization rate of raw materials and short reaction cycle , the effect of uniform distribution of elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
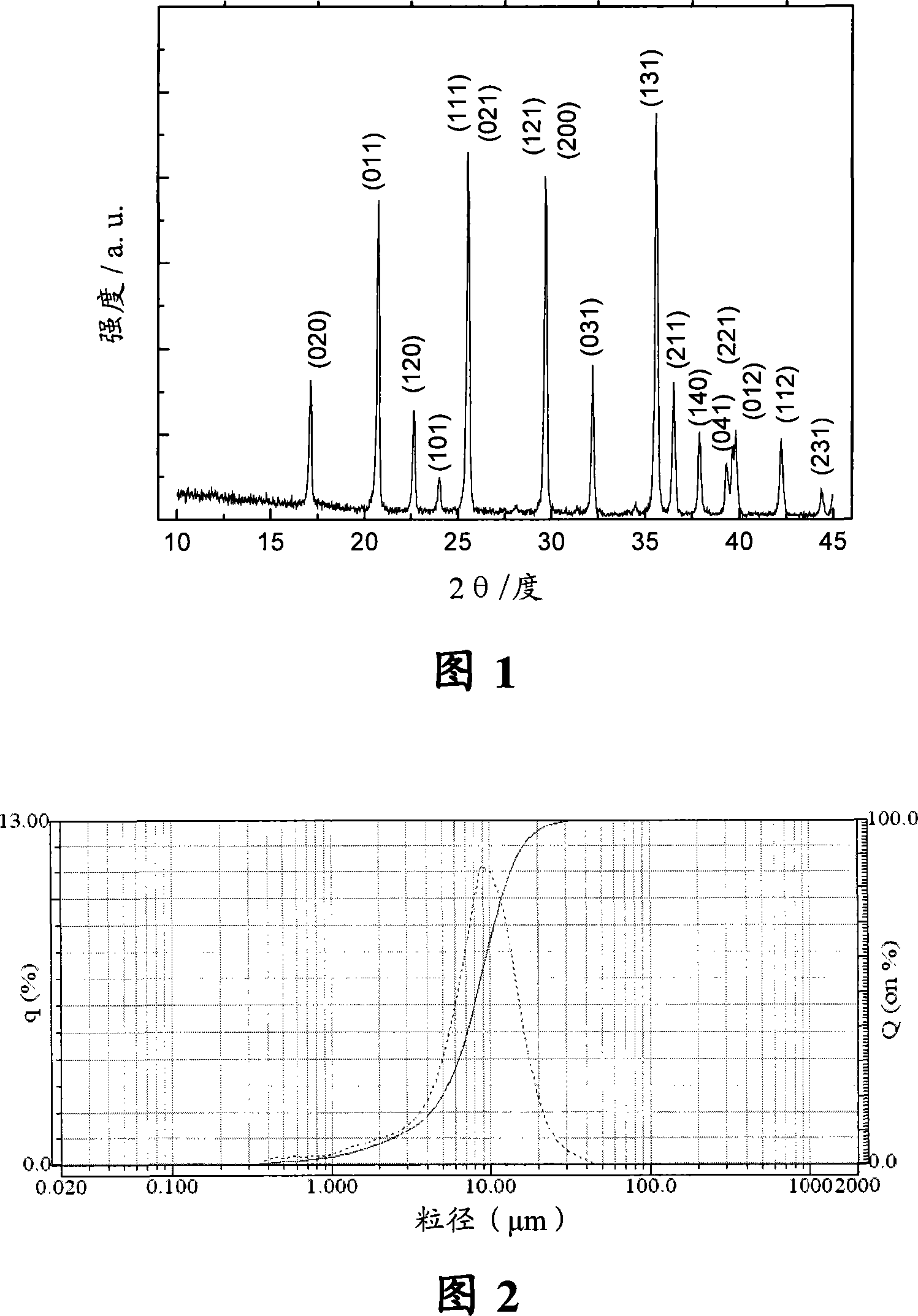
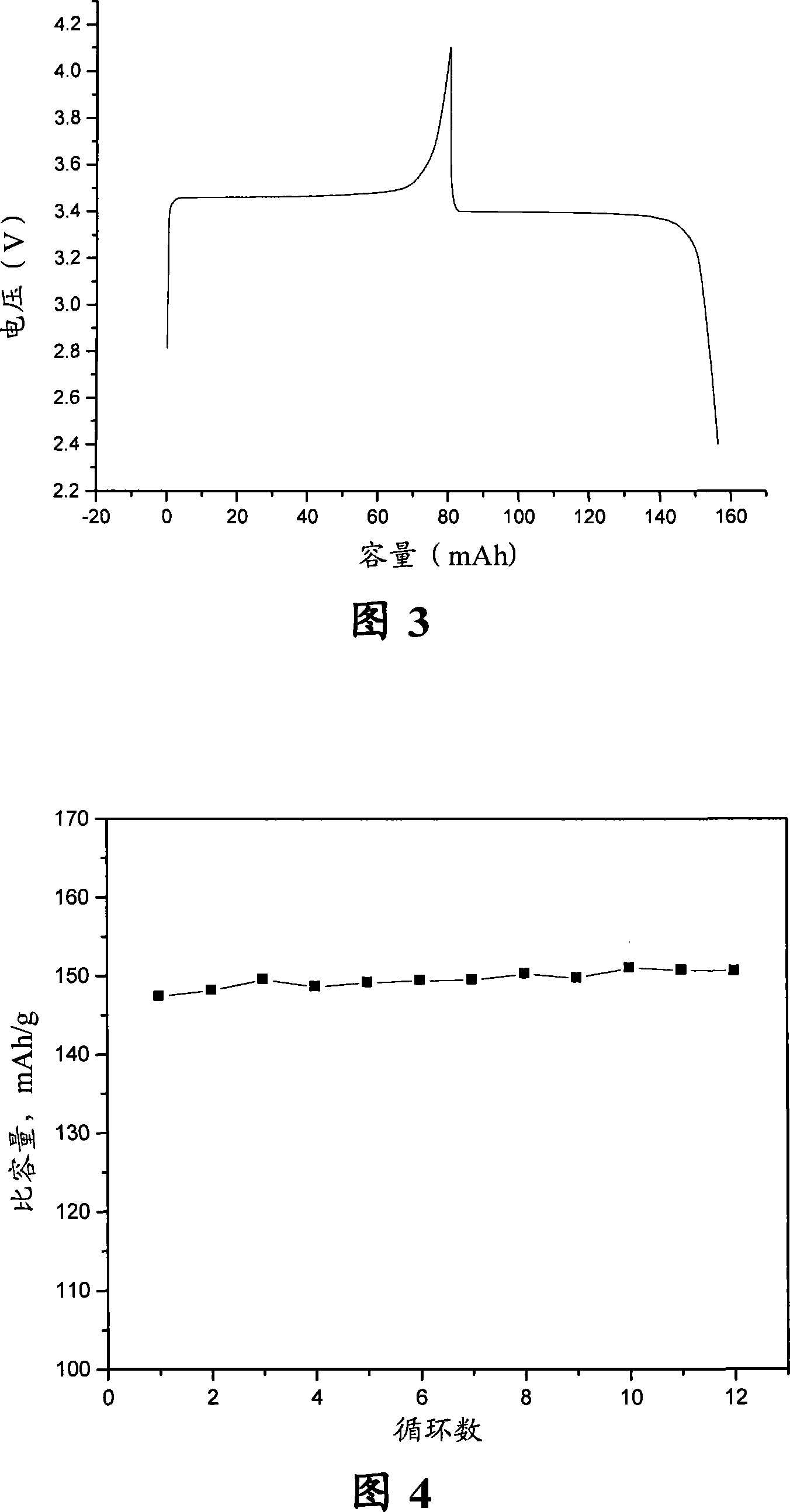
[0045] Get 0.9mol (50.4g) metallic iron (purity 99.8%) and put it in metal titanium blue as anode, graphite plate as cathode, with 34.74g lithium phosphate (0.3mol / L) and 42.57g phosphorus pentoxide (0.3mol / L) L) Dissolved in 1000ml of absolute ethanol as the electrolyte, the distance between the electrodes is 35mm, the electrolytic terminal voltage is 5V, and the current density is controlled at 600A / m 2 . After electrolysis, the electrolysis product was dried in a vacuum oven at 120° C. for 12 hours to obtain 142.1 g of dry powder precursor. Mix the powdery precursor with 1g of sucrose, then place it in a tube furnace, use nitrogen as a protective atmosphere, nitrogen flow rate 6L / min, raise the furnace temperature to 650 degrees at a heating rate of 5°C / min, and then After sintering for 24 hours, after cooling to room temperature, the product was ground through a 400-mesh sieve to obtain the lithium iron phosphate composite material LiFePO 4 / C, its carbon content is abou...
Embodiment 2
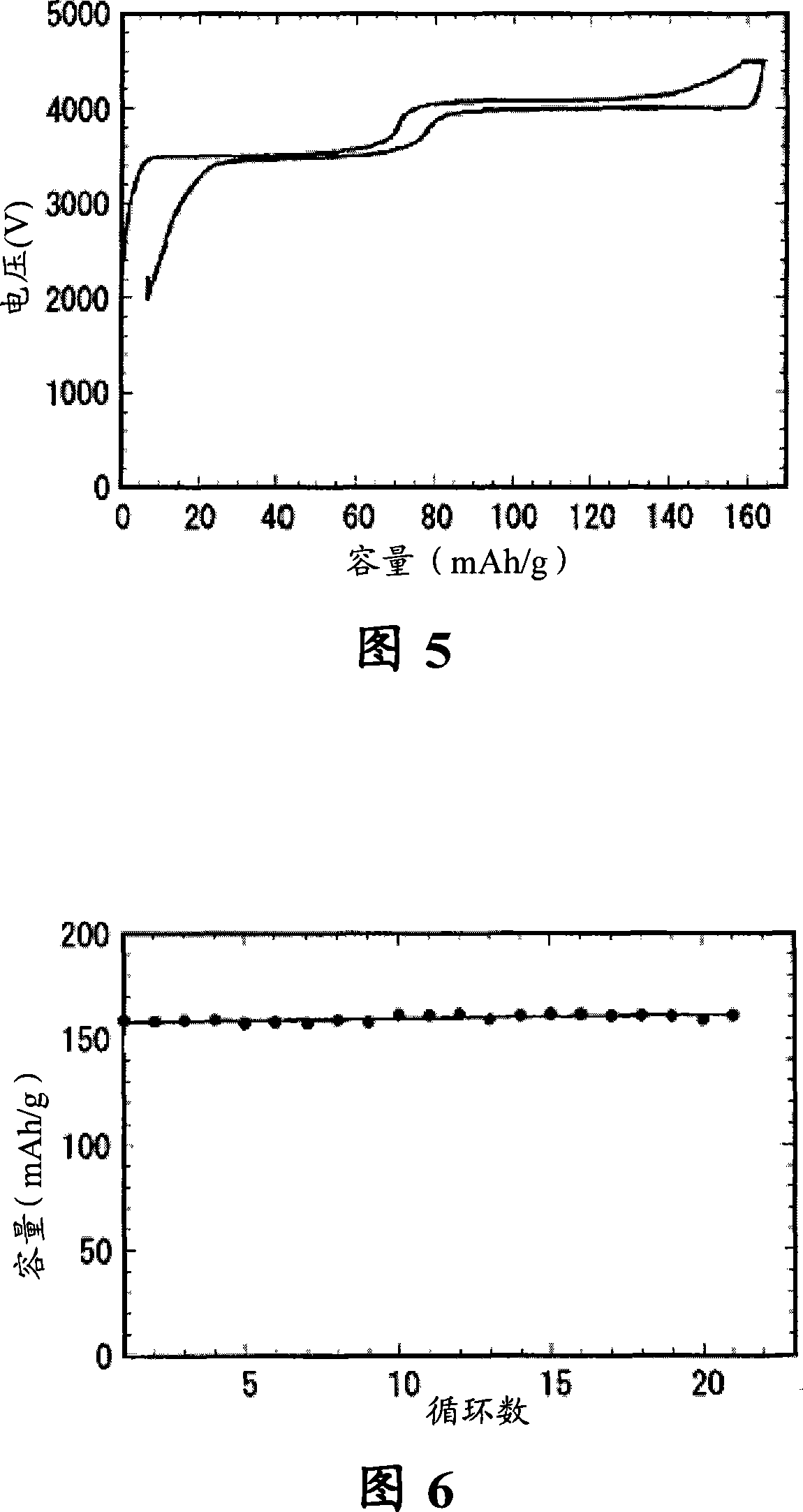
[0047] Get 0.36mol (20.16g) metal iron (purity 99.8%), and 0.54mol metal manganese (29.65g, purity 99.8%) are placed in metal titanium blue as anode, graphite plate is done cathode, with 34.74g lithium phosphate (0.3mol / L) and 42.57g of phosphorus pentoxide (0.3mol / L) were dissolved in 1000ml of absolute ethanol as the electrolyte, the distance between the electrodes was 35mm, the electrolytic terminal voltage was 5V, and the current density was controlled at 600A / m 2 , after electrolysis, the electrolysis product was placed in a vacuum oven and dried at 120° C. for 12 hours to obtain 141.6 g of dry powder precursor. Then the obtained powdery precursor was mixed with 1 g of sucrose, and the above-mentioned mixed material was placed in a tube furnace, and nitrogen was used as a protective atmosphere. The nitrogen flow rate was 6 L / min, and the furnace temperature was raised to 650 °C at a heating rate of 5 ° C / min. degree, and then sintered at 650 degree for 24h, after cooli...
Embodiment 3
[0049] Get 0.72mol (40.32g) metal iron (purity 99.8%), 0.18mol metal nickel (52.83g, purity 99.8%) and put it in metal titanium blue as anode, graphite plate as cathode, with 34.74g lithium phosphate (0.3mol / L) and .42.57g of phosphorus pentoxide (0.3mol / L) were dissolved in 1200ml of absolute ethanol as the electrolyte, the distance between the electrodes was 35mm, the electrolytic terminal voltage was 5V, and the current density was controlled at 600A / m 2 . After electrolysis, the electrolysis product was dried in a vacuum oven at 120° C. for 12 hours to obtain 142.5 g of dry powder precursor. Mix the powdery precursor with 1g of sucrose, then place it in a tube furnace, use nitrogen as a protective atmosphere, nitrogen flow rate 6L / min, raise the furnace temperature to 650 degrees at a heating rate of 5°C / min, and then After sintering for 24 hours, after cooling to room temperature, the product was ground through a 400-mesh sieve to obtain the lithium iron phosphate compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap