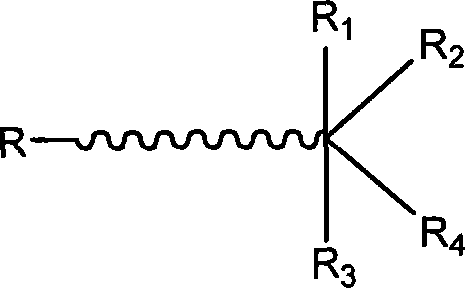
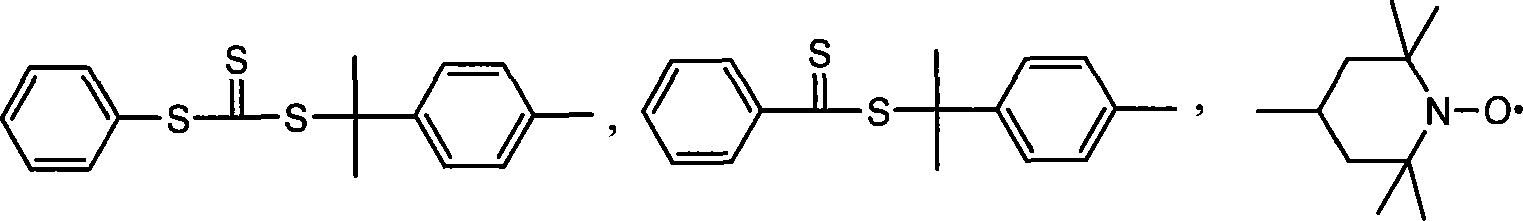
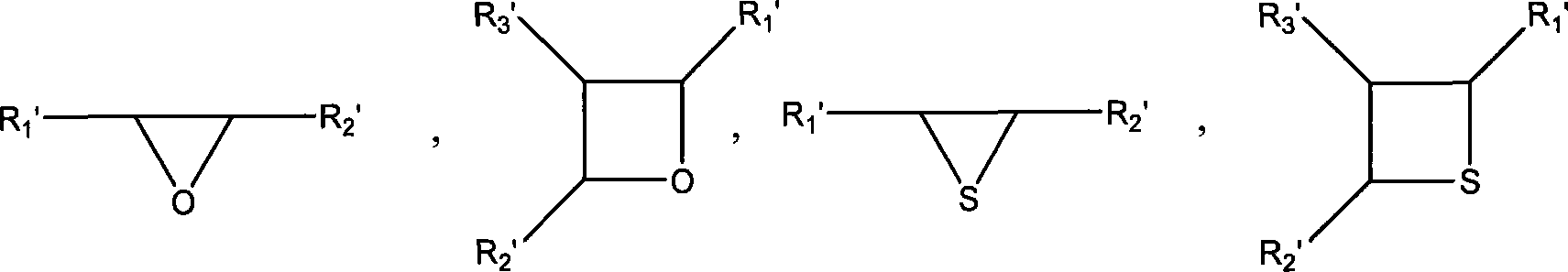
Polymer containing multiple functional groups at end of the same and preparation method thereof
A functional group, polymer technology, applied in the field of polymer material synthesis, can solve problems such as limited application and synthesis difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A 500mL ampoule was vacuum-degassed for 2 hours under infrared lamp baking, and after purging oxygen with nitrogen, 4mL of dry tetrahydrofuran, 120mL of cyclohexane and 10mL of styrene were added to the ampoule. The calculated amount of initiator (1.93 mL, 3.03 mmol) was then quickly injected with a syringe. The reaction was continued for 8 h at room temperature, and then, a mixed solution of 3 mL of 1-ethoxyethyl-2,3-epoxypropyl ether and 4 mL of tetrahydrofuran was added with a syringe, and methanol was added to terminate the reaction after overnight reaction. Concentrate the obtained solution, precipitate it in a large amount of methanol, and dry it under vacuum at 45°C for 12 hours to obtain a white polymer powder, that is, styrene whose end group contains a protected hydroxyl group and an "active" hydroxyl group. The reaction structure formula is:
[0041]
[0042] Add about 2.0 g of the above terminal-functionalized polystyrene into a 100 mL round-bottomed flas...
Embodiment 2
[0045] About 6.0 g of the functionalized polystyrene in Example 1 containing protected hydroxyl groups and "active" hydroxyl groups at the end groups was added to a clean 100 mL round bottom flask, and toluene was added for azeotropic water removal. Then add 150mL THF to dissolve the polymer, and add the polymer solution into a 250mL ampoule, and fill the system with nitrogen to make the internal and external pressures the same. Then add benzhydryl potassium solution under magnetic stirring to carry out protonation reaction to the hydroxyl group of the polymer end group, until the color of the system becomes benzhydryl potassium solution - dark brown, the ampoule bottle is placed in an ice-water bath, Let cool well. 1.5 mL of propyne bromide was added dropwise within 2 h via a syringe, and the reaction was continued for 24 h at room temperature. The salt in the system was separated by centrifugation, most of the solvent was removed by rotary evaporation from the supernatant, ...
Embodiment 3
[0050] Add about 2.0 g of the functionalized polystyrene in Example 2 containing alkyne groups and "active" hydroxyl groups at the same time into a clean 100 mL round bottom flask, add toluene to azeotropically remove water; then add 30 mL of refined anhydrous pyridine The polymer was dissolved, and the polymer solution was placed in an ice-water bath. Then under magnetic stirring, 0.50 mL of bromoisobutyryl bromide was added dropwise within 0.5 h, and the reaction was continued for about 24 h. The functionalized polystyrene of alkynyl and a bromine atom, its reaction structure is:
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com