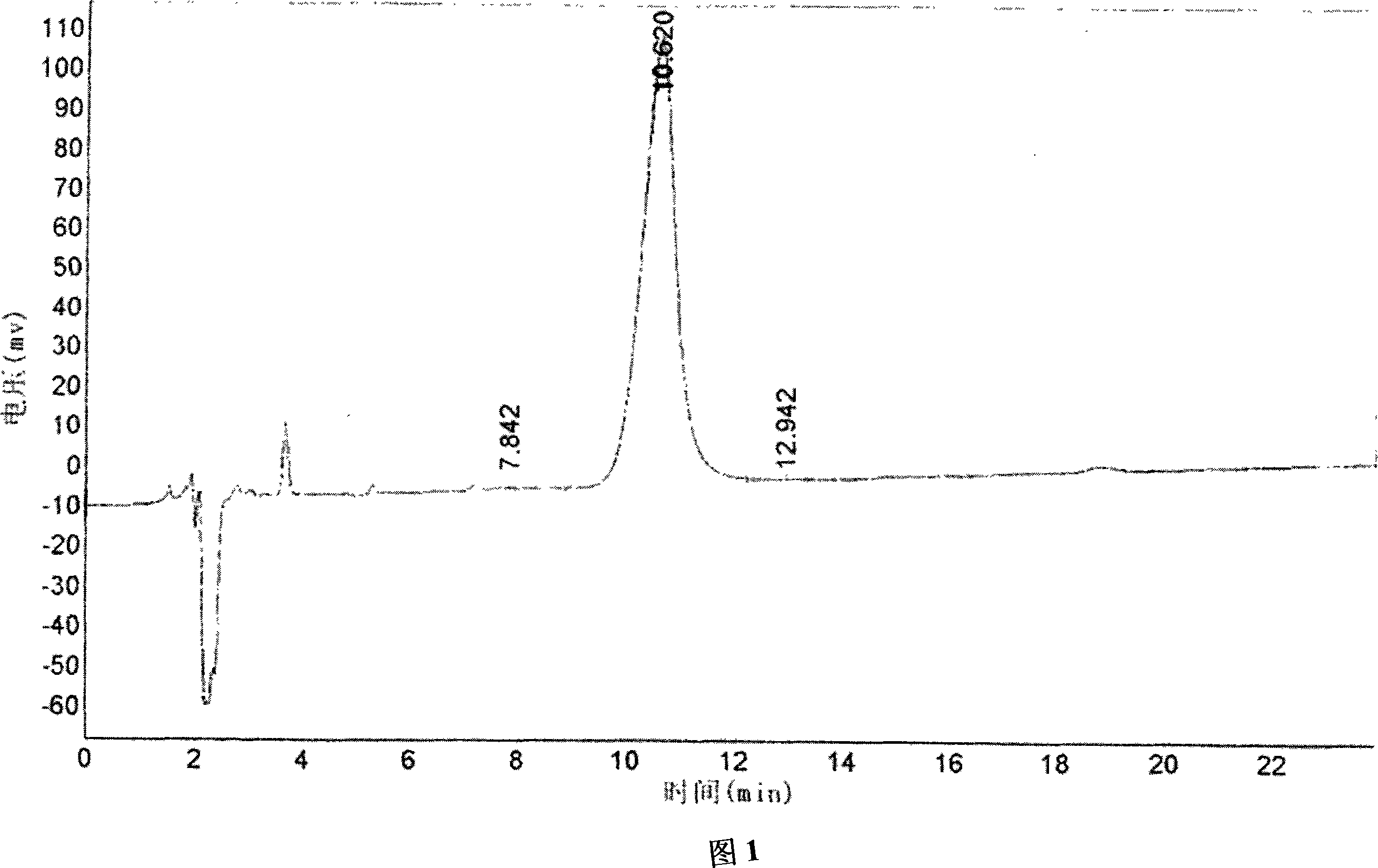
Method for manufacturing high purity monosialogangliosides
A ganglioside and monosialic acid technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as high phosphorus content, hidden safety hazards, and complicated processes, and achieve technological progress Simple, increase the relative amount, improve the effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In this example, fresh porcine brain tissue is used as raw material to prepare high-purity ganglioside GM1 dry powder, and the process steps are as follows:
[0031] (1) Preparation of brain tissue acetone powder
[0032] Clean fresh pig brain tissue, add -20 ℃ pre-cooling acetone, fresh pig brain tissue is measured in grams, pre-cooling acetone is measured in milliliters, fresh livestock brain tissue mass: volume of pre-cooling acetone = 1:10, using 5500rpm speed Homogenize for 30 minutes, collect the precipitate by gradient centrifugation, and vacuum-dry the precipitate to constant weight to obtain brain tissue acetone powder; the acetone in the centrifuged supernatant is recovered by vacuum distillation.
[0033] (2) Preparation of total gangliosides
[0034]The acetone powder prepared by step (1) is dissolved with the extract at normal pressure and room temperature, the acetone powder is measured in grams, the extract is measured in milliliters, the quality of the ...
Embodiment 2
[0047] In this example, fresh yak brain tissue was used as raw material to prepare high-purity ganglioside GM1 dry powder, and the process steps were as follows:
[0048] (1) Preparation of brain tissue acetone powder
[0049] Clean the fresh yak brain tissue, add -20 ℃ pre-cooled acetone, fresh pig brain tissue is measured in grams, pre-cooled acetone is measured in milliliters, fresh livestock brain tissue mass: volume of pre-cooled acetone = 1:8, using 4000rpm speed Homogenize for 30 minutes, collect the precipitate by gradient centrifugation, and vacuum-dry the precipitate to constant weight to obtain brain tissue acetone powder; the acetone in the centrifuged supernatant is recovered by vacuum distillation.
[0050] (2) Preparation of total gangliosides
[0051] The acetone powder prepared by step (1) is dissolved with the extract at normal pressure and room temperature, the acetone powder is measured in grams, the extract is measured in milliliters, the quality of the a...
Embodiment 3
[0065] In this example, fresh porcine brain tissue is used as raw material to prepare high-purity ganglioside GM1 dry powder, and the process steps are as follows:
[0066] (1) Preparation of brain tissue acetone powder
[0067] Clean fresh pig brain tissue, add -20 ℃ pre-cooling acetone, fresh pig brain tissue is measured in grams, pre-cooling acetone is measured in milliliters, fresh livestock brain tissue mass: pre-cooling acetone volume = 1:5, using 6000rpm speed Homogenize for 30 minutes, collect the precipitate by gradient centrifugation, and vacuum-dry the precipitate to constant weight to obtain brain tissue acetone powder; the acetone in the centrifuged supernatant is recovered by vacuum distillation.
[0068] (2) Preparation of total gangliosides
[0069] The acetone powder prepared by step (1) is dissolved with the extract at normal pressure and room temperature, the acetone powder is measured in grams, the extract is measured in milliliters, the quality of the ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com