Method for producing ammonium bisulfate and hydrogen chloride by using ammonium chloride and sulfuric acid
A technology of ammonium bisulfate and ammonium chloride, which is applied in the field of chemical technology and can solve problems such as affecting the reaction, excessive free acid, and affecting product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
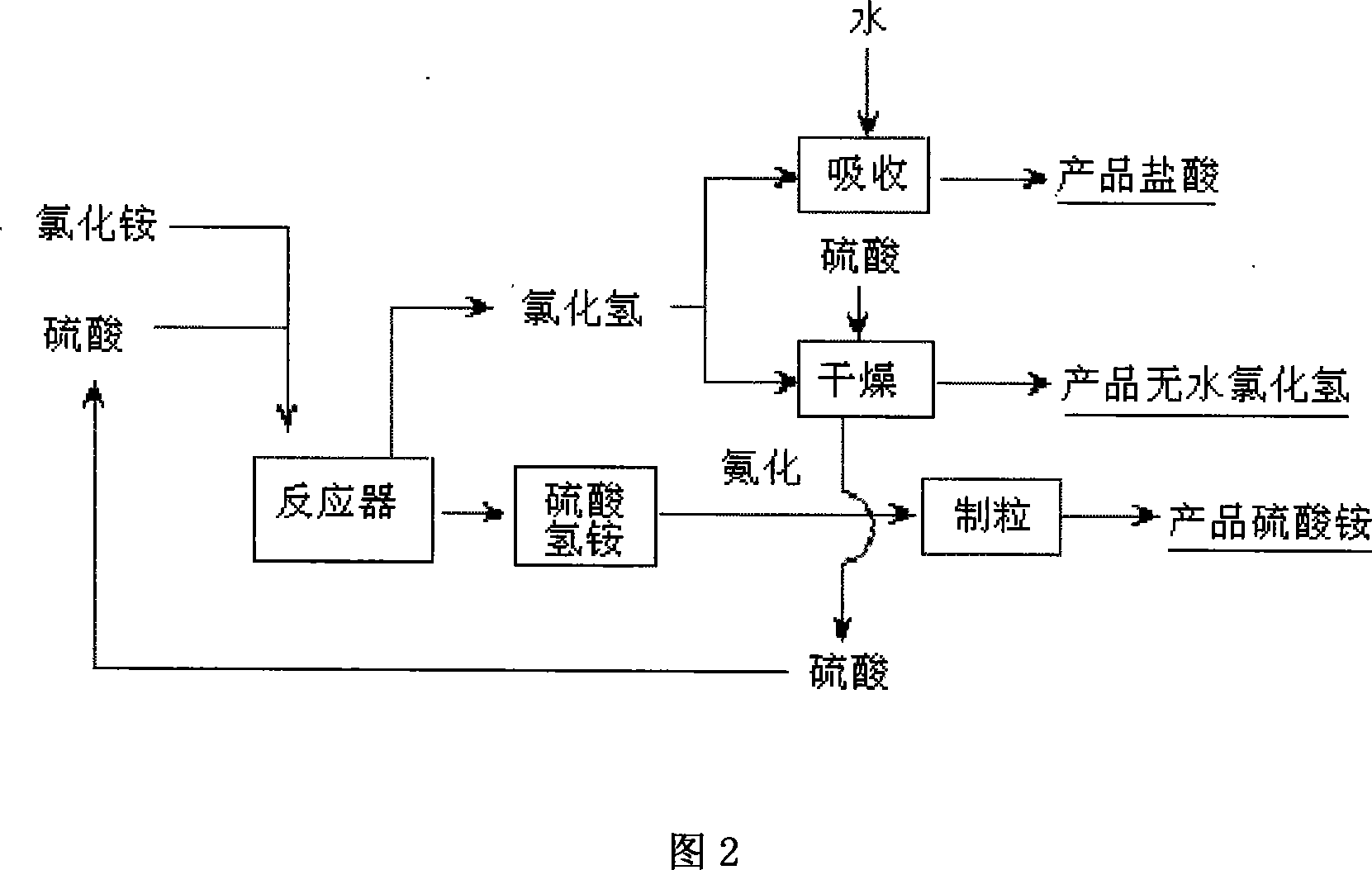
[0060] 5. Preparation of hydrochloric acid and anhydrous hydrogen chloride
[0061] The absorption of hydrogen chloride is completed in the packed tower, and the absorbent can be dilute acid (about 21% HCl) from the bottom of the desorption tower. The solubility of hydrogen chloride in water decreases with the increase of temperature. When the absorption temperature is 50°C (adiabatic absorption), 31% hydrochloric acid can be obtained, while when the absorption temperature is 30°C, 36% hydrochloric acid can be obtained. The packing absorption tower can choose steel lining tiles or phenolic impregnated graphite or rubber.
[0062] The purpose of desorption is to desorb HCl into a gaseous state by heating concentrated hydrochloric acid. HCl can be freed from concentrated hydrochloric acid at elevated temperatures, which is the desorption process.
[0063] In industry, the hydrochloric acid desorption tower and the dilute acid reboiler are actually a connected device. Concentr...
Embodiment 1
[0069] Embodiment 1: the preparation of ammonium bisulfate and hydrochloric acid
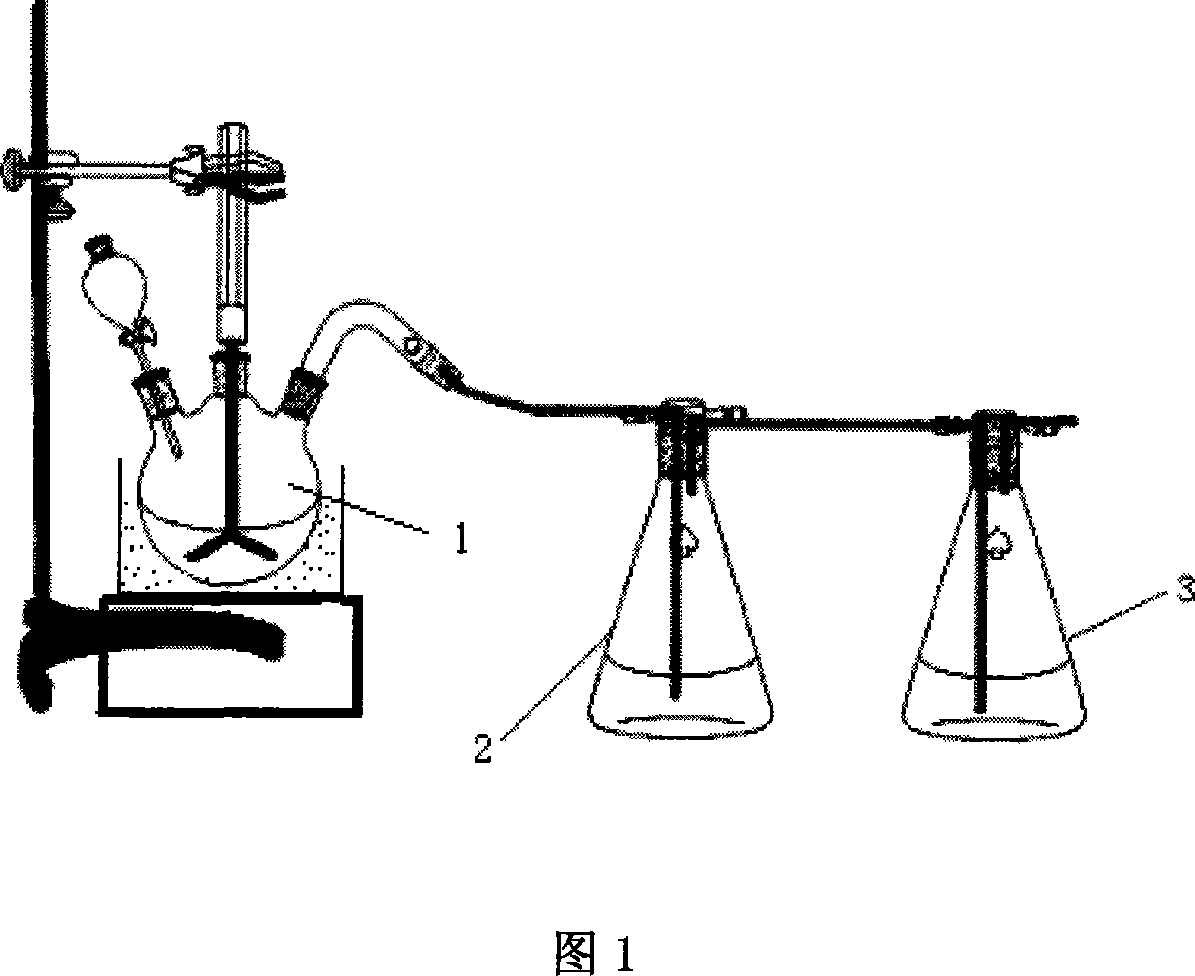
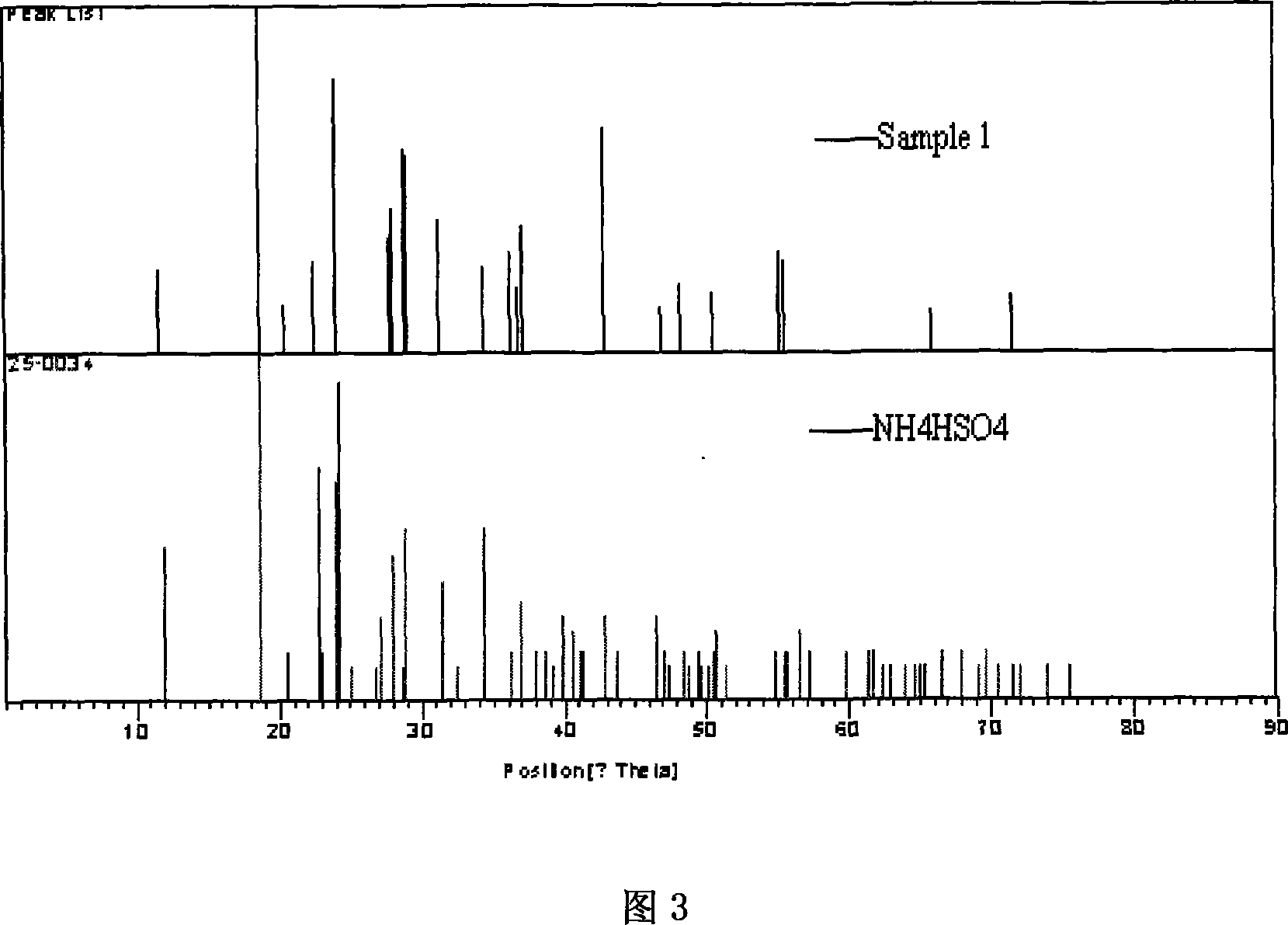
[0070] Weigh 100 grams of solid ammonium chloride into glass flask reactor 1, and gradually add industrial sulfuric acid (96wt%) of theoretically calculated amount under constant stirring. The reactor is connected to the absorption bottle 2 through a pipeline, and the hydrogen chloride gas generated is absorbed by the primary absorption bottle 2 and the secondary absorption bottle 3 and then emptied through the water circulation pump. The whole system maintains a slight negative pressure state. The temperature inside the glass flask reactor 1 is 120±3°C, and the reaction time is 30 minutes. After the reaction is completed, powdery ammonium bisulfate product is obtained. X-ray diffraction analysis, as shown in Figure 3, analysis result shows that product is ammonium bisulfate. The purity of ammonium bisulfate is 98wt%.
[0071] The first-level absorption bottle 2 is (31wt%) hydrochloric acid ...
Embodiment 2
[0072] Embodiment 2: the preparation of ammonium sulfate and hydrochloric acid
[0073] Add 1 kg of solid ammonium chloride and 1.91 kg of industrial sulfuric acid in a spiral stirring reactor in a parallel manner. The average residence time of materials in the reaction tank is 30min, and the reaction temperature is 120±10°C. Reactants and products are constantly entering and exiting. The discharged ammonium bisulfate product is ammoniated and granulated to make granular ammonium sulfate of about 1mm in size, with a purity of 99%.
[0074] The hydrogen chloride gas produced is sent to the water absorption tower through pipelines, and hydrochloric acid is generated through adiabatic absorption. The absorption tower is a packed tower, or it can be a type of absorption tower such as a valve tower. Hydrogen chloride gas enters the absorption tower from the lower part of the tower and forms a countercurrent flow with the water poured in from the upper part of the tower, passes t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com