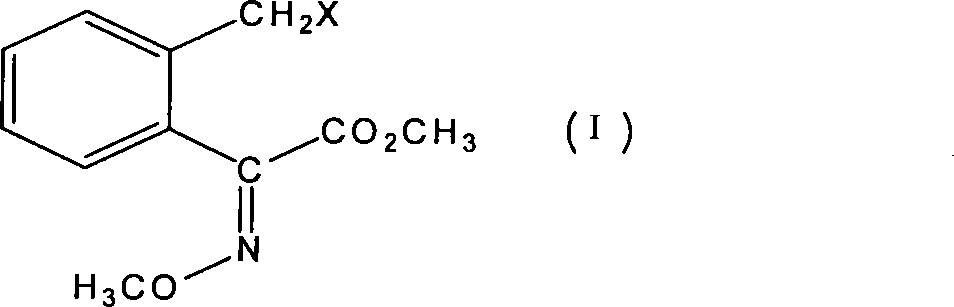Method for preparing bactericidal agent intermediate (E)-2-(2'-chloromethyl)phenyl-2-methoxy imino methyl acetate
A technology of methyl methoxyiminoacetate and o-methylol benzoic acid lactone is applied in the field of preparation of formula intermediates, and can solve the problem of device blockage, unsuitability, and interference with distillation and purification of o-chloromethyl benzoyl chloride and other problems to achieve the effect of improved safety, high yield and reduced risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
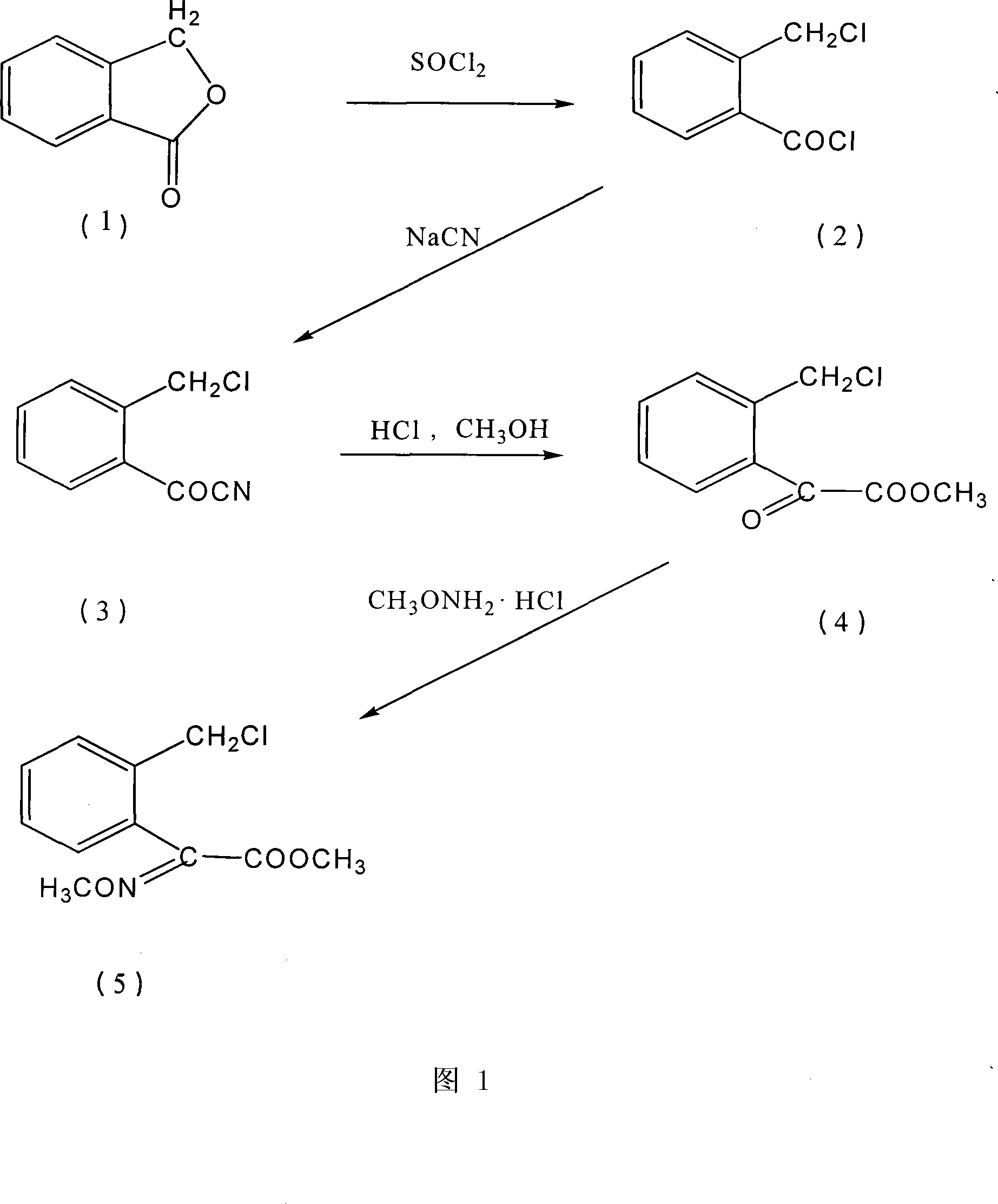
[0039] Embodiment 1, the preparation method of agricultural fungicide chemical intermediate (E)-2-(2'-chloromethyl)phenyl-2-methoxyiminoacetic acid methyl ester, with reference to Fig. 1:
[0040] Step 1, the preparation of o-chloromethylbenzoyl chloride
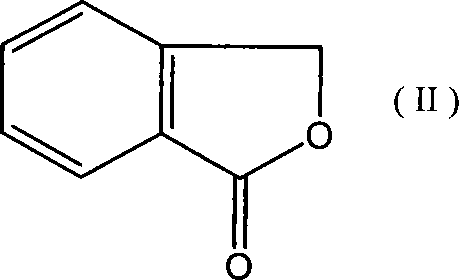
[0041] 271g (99%, 2.0mol) o-hydroxymethylbenzoic acid lactone (phthalide), 10.5g (99%, 0.1mol) trimethyl borate, 28.5g (99% 0.1mol) triphenylphosphine oxide were added to In a 500ml reaction flask, heat it to 130°C under stirring, and add 324.5g (99%, 2.7mol) of thionyl chloride dropwise to the reaction flask for 3 to 5 hours. After the addition is complete, the mixture is reacted at 130°C for 5 hours. The reaction effluent was distilled under reduced pressure to obtain 366.4 g of the product; the purity was 98% (GC), and the yield was 95%.
[0042] Step 2, the preparation of o-chloromethyl benzoyl cyanide
[0043]Add 120g (98%, 2.4mol) of sodium cyanide and 470ml of water into a 3000ml reaction flask, dissolve the sodium ...
Embodiment 2
[0050] Embodiment 2 is basically the same as Embodiment 1, but has the following changes:
[0051] Step 1, the preparation of o-chloromethylbenzoyl chloride
[0052] Add 271g (99%, 2.0mol) o-hydroxymethylbenzoic acid lactone (phthalide), 3.8g (99%, 0.06mol) boric acid, 17g (99%, 0.06mol) triphenylphosphine oxide to a 500ml reaction bottle 324.5 g (99%, 2.7 mol) of thionyl chloride was added dropwise to the reaction flask in 3 to 5 hours, and after the addition was completed, the mixture was reacted at 130°C for another 5 hours, and the reaction discharge Distillation under reduced pressure gave 366 g of the product; the purity was 97% (GC), and the yield was 94%.
[0053] Step 2, the preparation of o-chloromethyl benzoyl cyanide
[0054] Add 162.5g (96%, 2.4mol) of potassium cyanide and 460ml of water into a 3000ml reaction flask, dissolve potassium cyanide into an aqueous solution under stirring, then add 1544g of toluene, 23g (99%, 0.1mol) of benzyl trichloride Ethyl ammo...
Embodiment 3
[0055] Embodiment 3 is basically the same as Embodiment 1, but has the following changes:
[0056] Step 1, the preparation of o-chloromethylbenzoyl chloride
[0057] 271g (99%, 2.0mol) o-hydroxymethylbenzoic acid lactone (phthalide), 10.5g (99%, 0.1mol) trimethyl borate, 26.5g (99%, 0.1mol) triphenylphosphine were added to In a 500ml reaction flask, heat it to 130°C under stirring, and add 324.5g (99%, 2.7mol) of thionyl chloride dropwise to the reaction flask for 3 to 5 hours. After the addition is complete, the mixture is reacted at 130°C for 5 hours. The reaction effluent was distilled under reduced pressure to obtain 370.5 g of the product; the purity was 97% (GC), and the yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com