Chitose/polyglycol blending medicine film and its preparing method as well as purpose
A technology of polyethylene glycol and chitosan, which is used in medical science, absorbent pads, bandages, etc., can solve the problems of unsatisfactory comprehensive performance of pure chitosan, achieve excellent drug release performance, wide application prospects, and improved performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
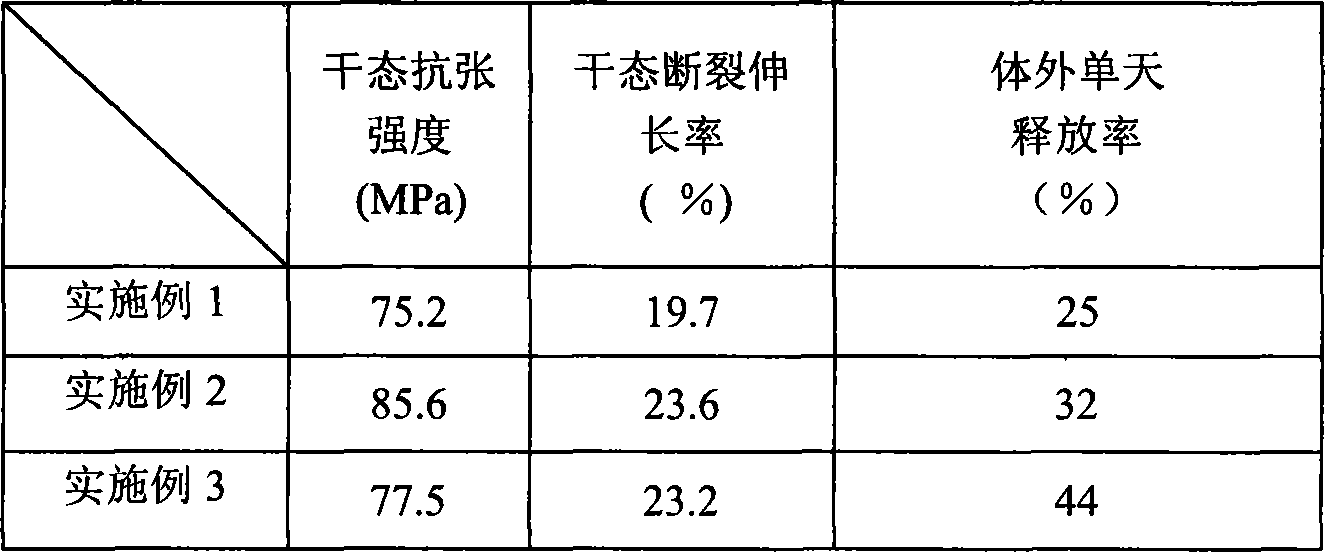
[0010] Embodiment 1: get 4g deacetylation degree and be 87%, viscosity-average molecular weight is 8.5 * 10 5 Chitosan is dissolved in the acetic acid solution of 100ml2% (mass ratio) to obtain chitosan solution; Get 0.15g viscosity-average molecular weight and be 6000, analytically pure polyethylene glycol is dissolved in 4ml distilled water to obtain polyethylene glycol solution. Fully mix the above two solutions with 0.4g ciprofloxacin hydrochloride, filter, degass under reduced pressure, use 1% to 3% (mass ratio) sodium tripolyphosphate aqueous solution as the coagulation solution, evaporate at room temperature by salivation Preparation of chitosan / polyethylene glycol blended drug-loaded film. Its mechanical properties and release properties are listed in Table 1.
Embodiment 2
[0011] Embodiment 2: Get 4g chitosan and dissolve in the acetic acid solution of 100ml 2% (mass ratio) to obtain chitosan solution; Get 0.3g polyethylene glycol and dissolve in 8ml distilled water to obtain polyethylene glycol solution. Fully mix the above two solutions with 0.4g ciprofloxacin hydrochloride, filter, degass under reduced pressure, use 1% to 3% (mass ratio) sodium tripolyphosphate aqueous solution as the coagulation solution, evaporate at room temperature by salivation Preparation of chitosan / polyethylene glycol blended drug-loaded film. Its mechanical properties and release properties are listed in Table 1.
Embodiment 3
[0012] Embodiment 3: Get 4g chitosan and dissolve in the acetic acid solution of 100ml 2% (mass ratio) to obtain chitosan solution; Get 0.6g polyethylene glycol and dissolve in 15ml distilled water to obtain polyethylene glycol solution. Fully mix the above two solutions with 0.4g ciprofloxacin hydrochloride, filter, degass under reduced pressure, use 1% to 3% (mass ratio) sodium tripolyphosphate aqueous solution as the coagulation solution, evaporate at room temperature by salivation Preparation of chitosan / polyethylene glycol blended drug-loaded film. Its mechanical properties and release properties are listed in Table 1.
[0013] The salivation evaporation method used in the above embodiments is a prior art, which has been described in detail in the existing literature, so the salivation evaporation method will not be repeated in each embodiment.
[0014] Table 1
[0015]
[0016] As can be seen from the data in Table 1, the chitosan / starch blended drug-loaded film pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com