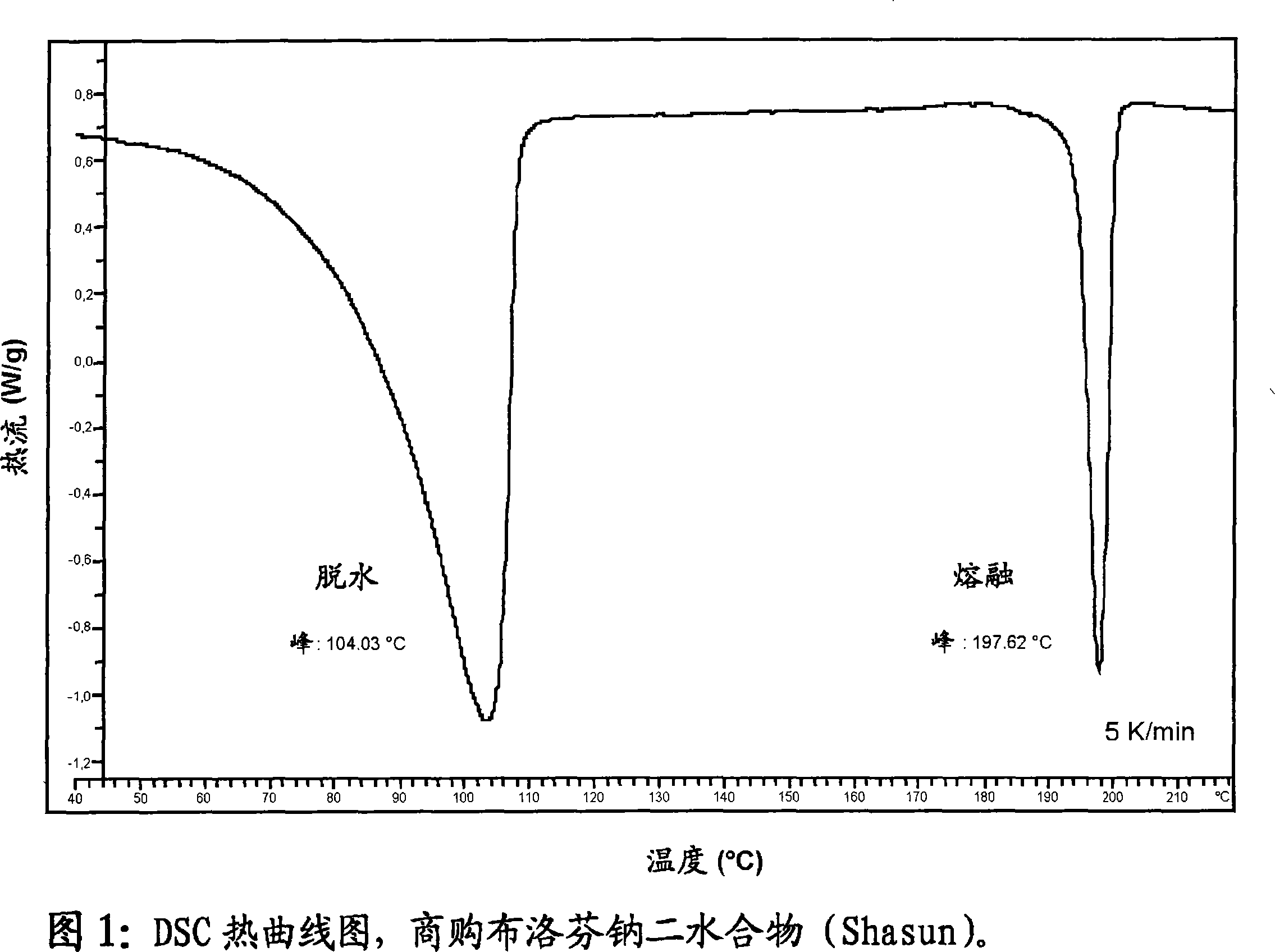
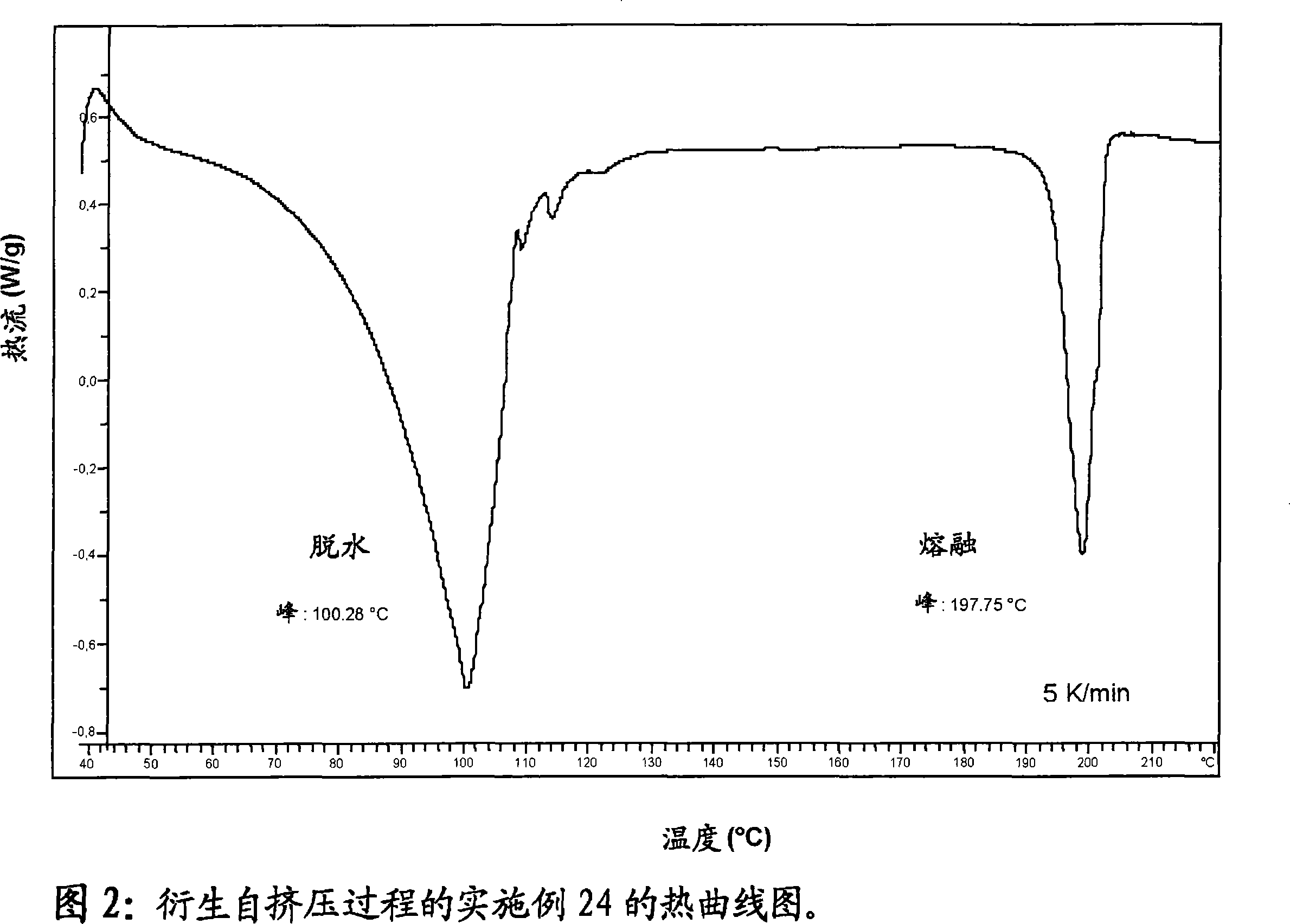
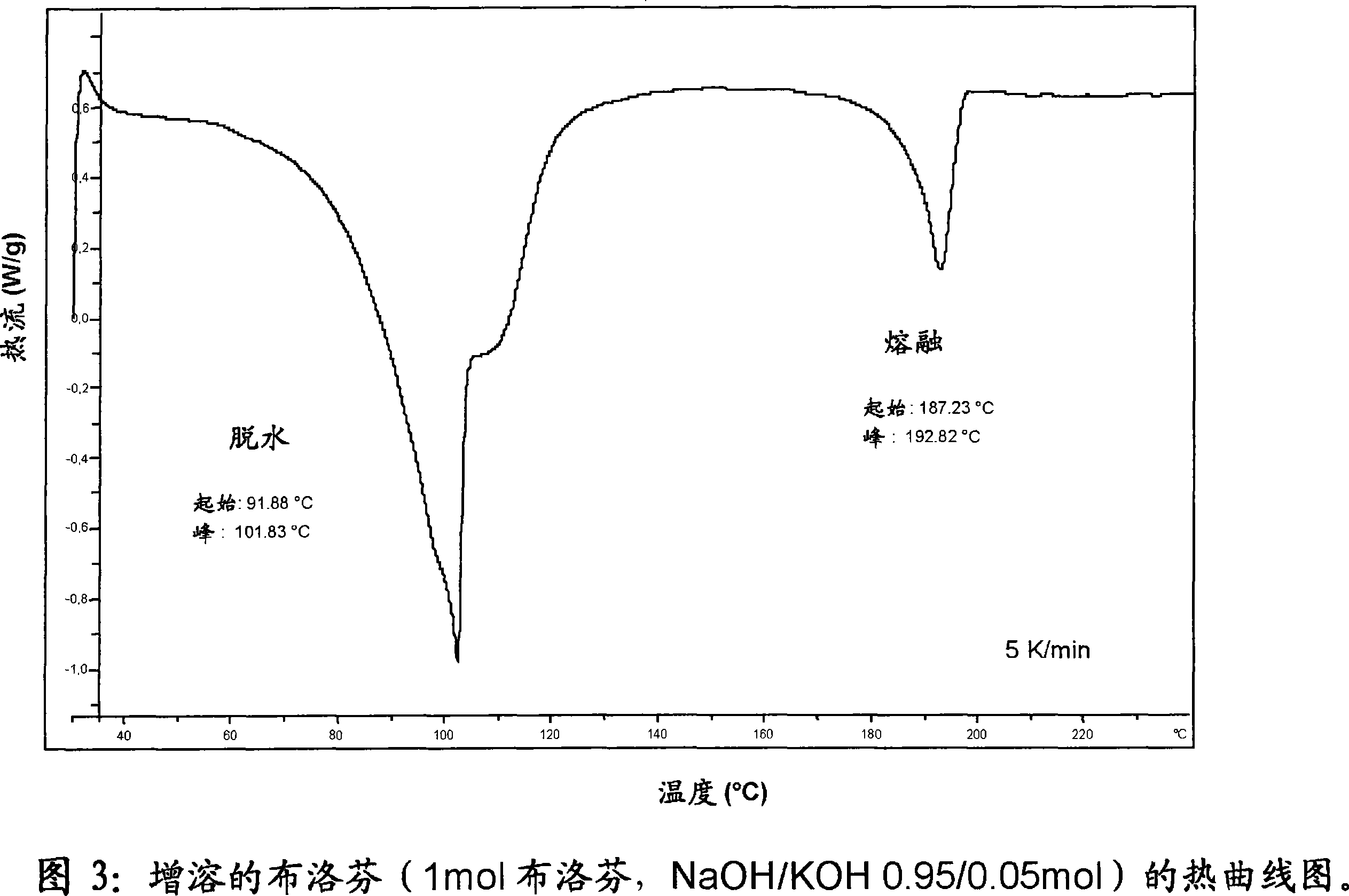
Solubilized ibuprofen
A technology of mixture and potassium carbonate, applied in the direction of anhydride/acid/halide active ingredients, microcapsules, capsule delivery, etc., can solve the problems that do not mention the importance of water content ibuprofen dehydrate or hydrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0134] The examples outlined in Table 1 were carried out in a heatable and coolable mixing vessel.
[0135]
Example
base and excipients a)
heating b)
product
temperature c)
notes d)
1
0.95 mole NaOH
2.0% Mannitol
(ambient temperature)
81℃
Vigorously exothermic; add 1 mole
Water; granular powder, very weak
2
1.0 mol Na 2 CO 3
0.4 mol KCl
8% Povidone K25
38℃
41℃
Add 0.5 moles of water before solubilization
And add 1.5 molar after solubilization
Er water; granule powder, good
compressibility of
3
1.0 mole K 2 CO 3
65℃
86℃
Add 0.2 moles of water, hygroscopicity
fine powder, very good
Compressibility, v...
Embodiment 16
[0146] a) 6.18 kg (30 moles) of ibuprofen, 2.25 kg (30 moles) of glycine and 1.2 kg (30 moles) of ground sodium hydroxide were mixed in a mixing vessel. The temperature of the vigorously stirred mixture was raised to 68° C. over 20 minutes while the mixture was transformed into a viscous mass. A 1 g sample of pellets dissolved in 100 mL of 37°C water within 35 seconds, thus indicating that solubilization was complete. 540 g of water were added to the warm mass within 5 minutes, and the mass was converted to coarse granules within 10 minutes with slow stirring. After 15 minutes at 60° C., the granules were sieved through a sieve with a mesh size of 1.5 mm. The obtained granules (granule A) had a loss on drying of 11.2% w / w at 105°C for 30 minutes. Granular samples stored in a desiccator at 25°C and 75% relative humidity for 2 months absorbed only 0.3% w / w of water. A portion of the granules was dried in a drying oven at 60°C until the loss on drying (105°C, 30 minutes) was 6...
Embodiment 17
[0154] Mix 206 kg (1000 moles) of ibuprofen with 16 kg (400 moles) of ground sodium hydroxide, 47.7 kg (450 moles) of sodium carbonate, 13.8 kg (100 moles) of potassium carbonate, 10 kg of povidone K25 and 7 kg of sucrose monohard The fatty acid esters are mixed vigorously in a mixing vessel. The temperature of the mixture was raised to about 50°C and within 30 minutes slightly sticky granules formed. 1 g of granule sample was dissolved in 100 mL of 37° C. water within 25 seconds. The solubilized granules were transferred to a fluid bed granulator and sprayed in 100 liters of water at an inlet air temperature of about 30°C. The moisture content of the granules obtained was 8.8% w / w, measured according to the loss on drying method at 70° C. for 30 minutes. The granules were stored for 3 months at 25°C and 75% relative humidity, and their water uptake was 2.8% (w / w).
[0155] The granules obtained are mixed with 1.5% magnesium stearate in a fluidized bed granulator and compre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| grain size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com