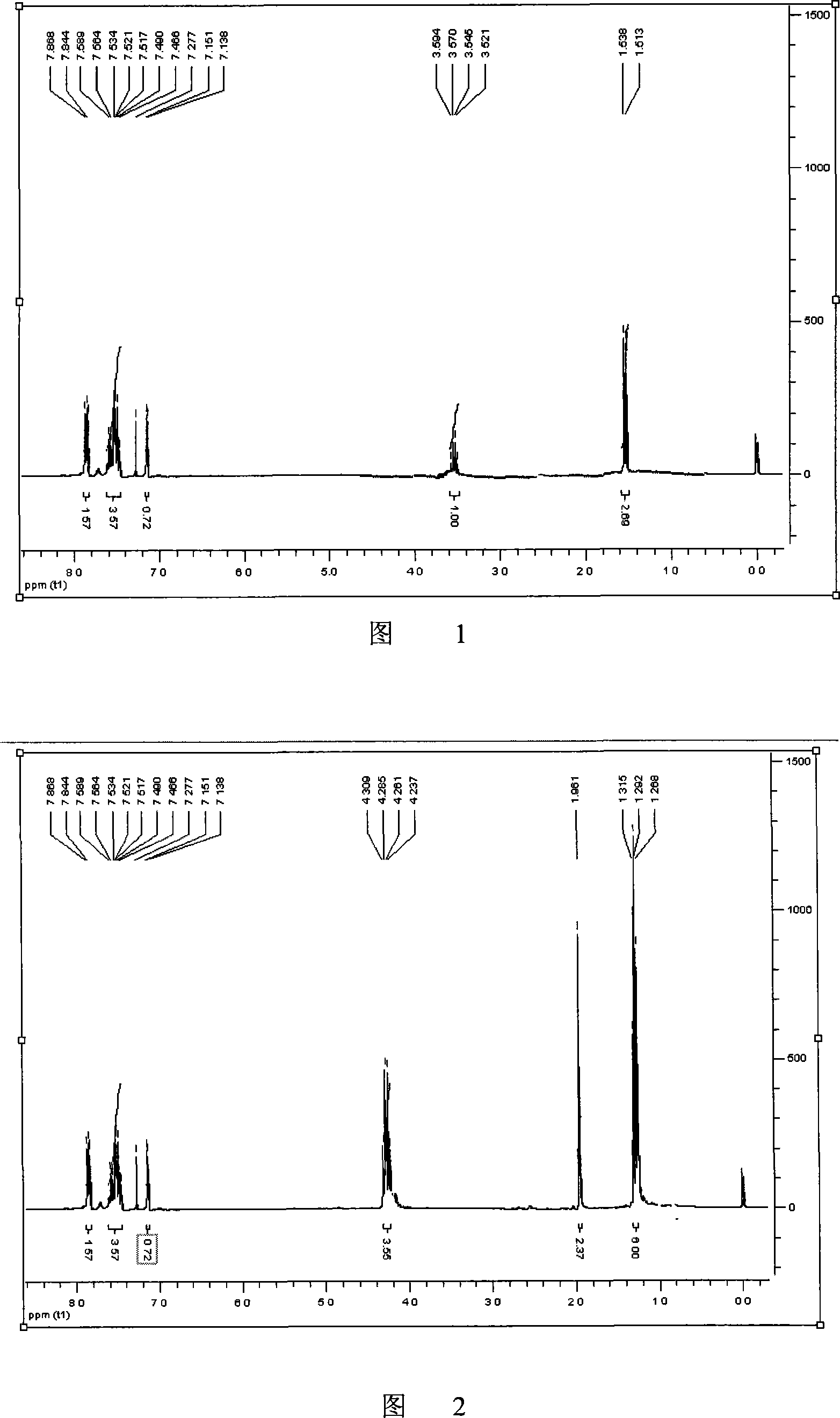
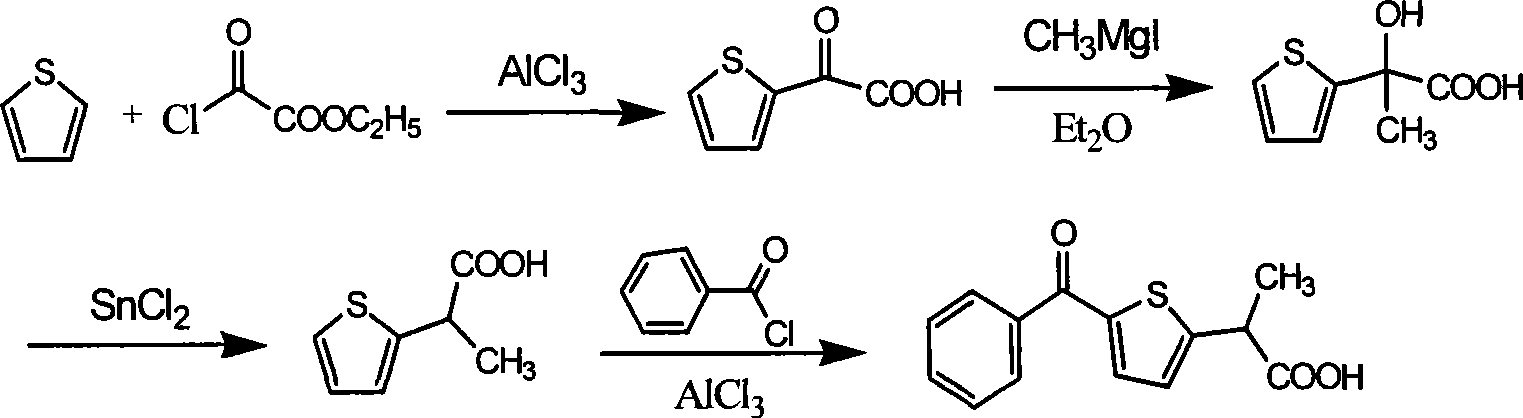
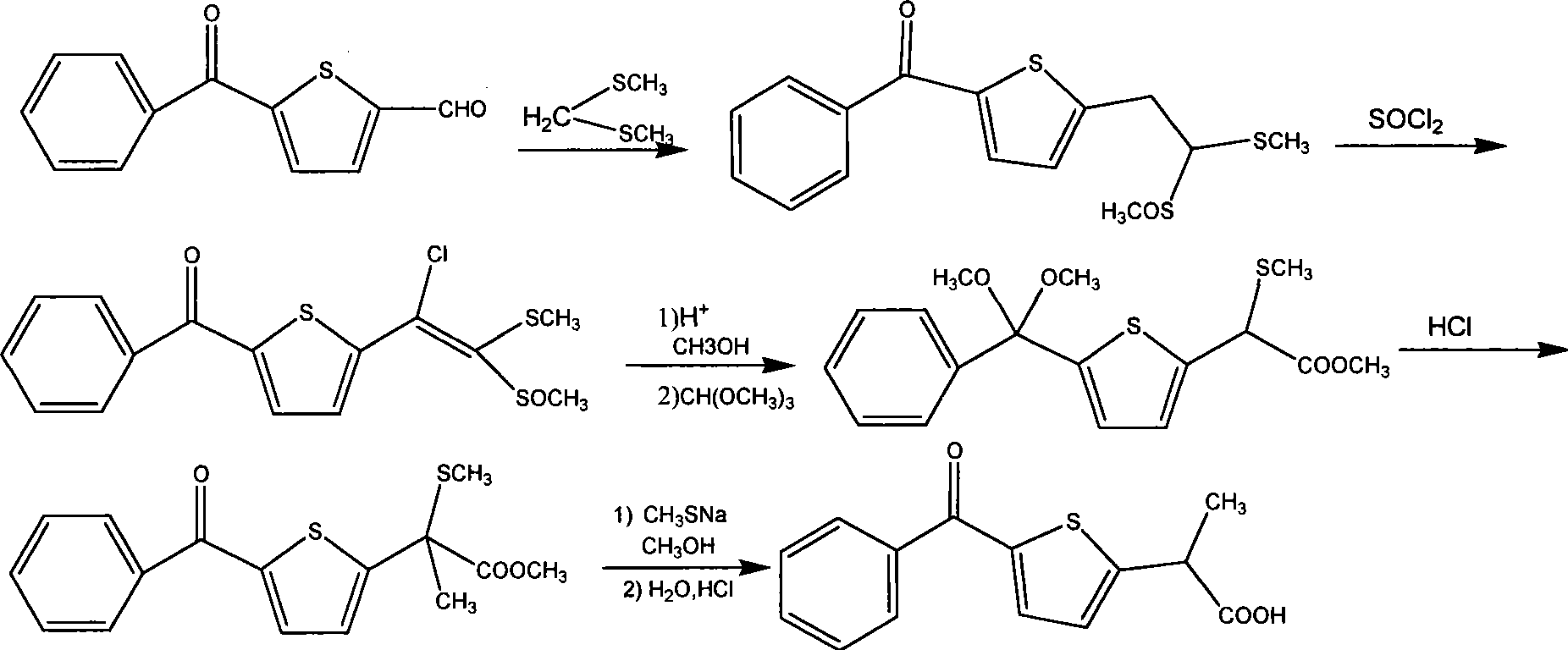
Method for synthesizing non-steroidal antiphlogiston tiaprofenic acid
A non-steroidal anti-inflammatory drug and a synthesis method technology, applied in the direction of organic chemistry and the like, can solve problems such as pollution, and achieve the effects of simple process route, short circuit and avoiding pollution problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0034] The preparation of embodiment 1.2-thiophene benzophenone:
[0035] In a three-necked flask equipped with mechanical stirring, a condenser and a thermometer, place anhydrous AlCl 3 10.0g, CS 2 30.0 g. Cool the suspension to 15-25°C, and dissolve thiophene 6.0g (71mmol) and benzoyl chloride 10.5g (75mmol) in 22.5g CS under stirring within 3.5h 2 The resulting solution was added to the flask through a condenser. The solution was allowed to warm to room temperature and stirring was continued for 3 more h, the mixture was left overnight. Then the mixture was refluxed on a water bath for 3.5h, cooled, poured on ice and extracted with 50mL ether. The extract was sequentially washed with 30mL saturated NaHCO 3 and 30mL of aqueous phase, and then in anhydrous CaCl 2 Dry on top. Ether was distilled off in a water bath, and the residue was distilled under reduced pressure to collect 11.7 g of 152-156°C / 8mmHg fraction with a yield of 87.3%. It solidified after cooling, with...
Embodiment 2
[0036] Example 2. Preparation of 2.5-benzoyl-α-methyl-2-thiophene malonate diethyl ester by ultrasonic method:
[0037] Add 0.94g (5mmol) of 2-thiophene benzophenone, 0.87g (5mmol) of diethyl methylmalonate, 0.82g (10mmol) of anhydrous NaOAc, Mn(OAc) to the reaction flask successively 3 4.56g (15mmol) and 30ml of acetic acid. Under the protection of nitrogen, it was heated to 80° C. under power ultrasonic radiation, and reacted for 5 h. After the solution was cooled to room temperature, 20 mL of ethyl acetate was added, and the precipitated solid was filtered. The filtrate was successively washed with 20ml of 10% NaHCO 3 solution, washed with 20mL saturated brine and washed with anhydrous MgSO 4 dry. The solvent was distilled off to obtain 1.65 g of light yellow oily liquid with a yield of 91.7%.
Embodiment 3
[0038] Example 3. Preparation of 5-benzoyl-α-methyl-2-thiophene malonate diethyl ester by non-ultrasonic method:
[0039] Add 1.88g of 2-thiophene benzophenone, 1.74g of diethyl methylmalonate, 1.60g of anhydrous NaOAc, Mn(OAc) to the reaction flask successively 310.56g and 50ml of acetic acid. Heated to 80°C and reacted for 16h. After the solution was cooled, 50 mL of benzene was added and filtered. The filtrate was sequentially washed with 10% NaHCO 3 solution, after washing with saturated brine, with anhydrous MgSO 4 dry. The solvent was evaporated and recrystallized to obtain 3.10 g of light yellow oily liquid with a yield of 86.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com