Application of king cobra toxin protease inhibitor and its derivatives
A protease inhibitor, snake venom technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: Separation, purification and activity determination of king cobra venom protease inhibitor
[0105] 1. Separation and purification
[0106] During the separation and purification process, the inhibitory activity against trypsin and chymotrypsin was detected to track:
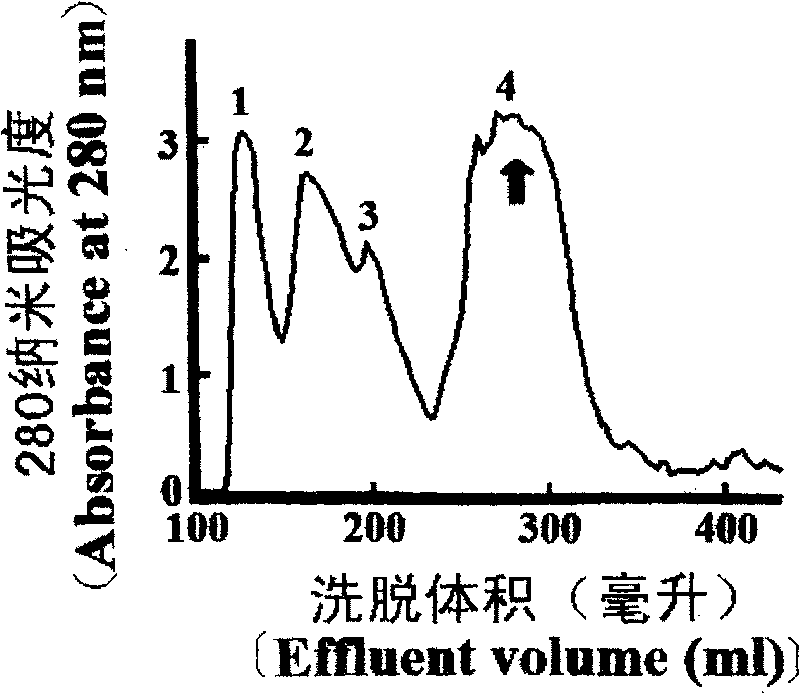
[0107] The first step, molecular sieve Sephadex G-50 gel filtration: King cobra crude venom 0.5g is dissolved in 3ml phosphate buffer (Na 2 HPO 4 -NaH 2 PO 4 , pH 5.8, containing 0.1M NaCl), through Sephadex G-50 gel. G-50IV peak has dual inhibitory activity on trypsin and chymotrypsin, attached figure 1 arrow peak.
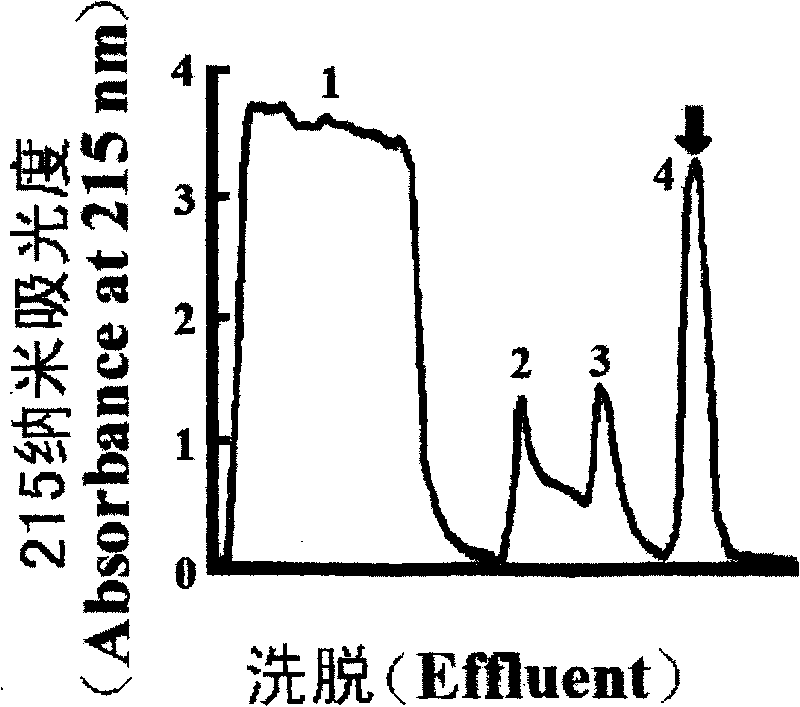
[0108] In the second step, the G-50IV peak was further separated and purified by trypsin affinity chromatography. Has dual inhibitory activity against trypsin and chymotrypsin in the hydrochloric acid elution peak, attached figure 2 arrow peak.
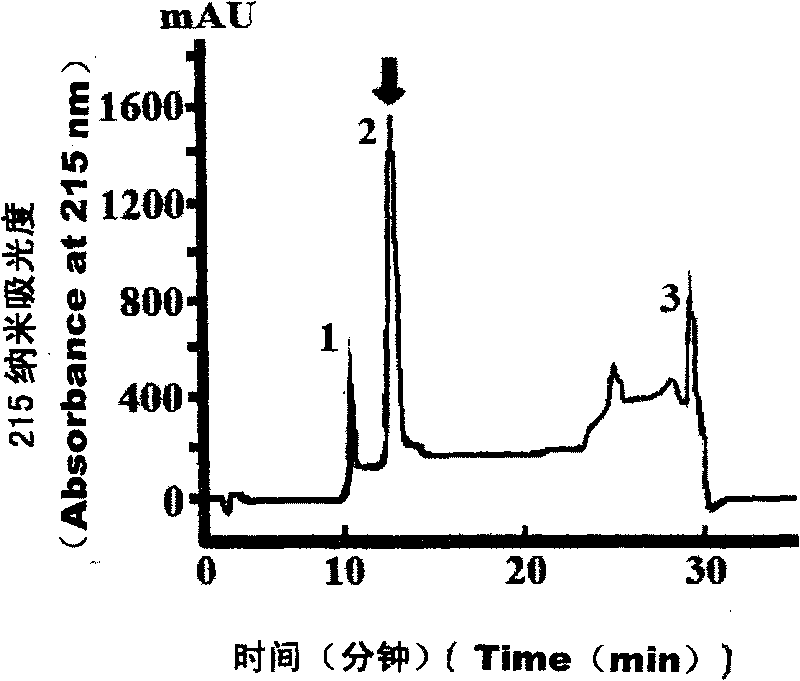
[0109] The third step, HPLC-RP-C 18 Purification of Trypsin Affinity Chromatography Eluted Peaks by Hydrophobic Chromatogr...
Embodiment 2
[0114] Embodiment 2: Structural determination and molecular cloning of king cobra venom protease inhibitor
[0115] 1. Structure determination:
[0116] Determination of N-terminal partial amino acid sequence
[0117] The purified main peak protein was subjected to Edman degradation method on an automatic protein sequencer (476A type, product of Applied Biosystems, USA), and the sequence of the N-terminal 20 amino acid residues of the main peak protein was determined.
[0118] Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry (MALDI-TOF-MS) Detection
[0119] The purified main peak protein was determined by MALDI-TOF-MS (Bunker) to determine its precise molecular weight.
[0120] 2. Molecular cloning:
[0121] Construction of cDNA Library of King Cobra Venom Gland
[0122] 1) Extraction of total RNA from king cobra venom glands: three days after taking venom from live king cobras, put them into liquid nitrogen for 4 hours and then peel off the venom gland t...
Embodiment 3
[0127] Embodiment 3: the prokaryotic expression of recombinant king cobra venom protease inhibitor
[0128] Expression of recombinant protein in Escherichia coli
[0129] 1. The cloned king cobra venom trypsin / chymotrypsin dual-functional inhibitor (OH-TCI) was amplified by PCR, and the 5'-terminal primer used corresponds to the N-end of the mature polypeptide, and the 3'-primer corresponds to the mature polypeptide The C-terminus of the polypeptide contains a restriction endonuclease Hind III cleavage site and protected nucleotides. PCR amplification was performed using high-fidelity DNA polymerase Pyrobest, a product of Treasure Bioengineering (Dalian) Co., Ltd. After the product was recovered, it was digested with restriction endonuclease Hind III, and then ligated with the expression vector (pMAL-p2X) cut with restriction endonuclease Xmn I and Hind III for blunt-sticky ends, and transformed into E. coli DH5α in cells. The expression vector pMAL-p2X was purchased from N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com