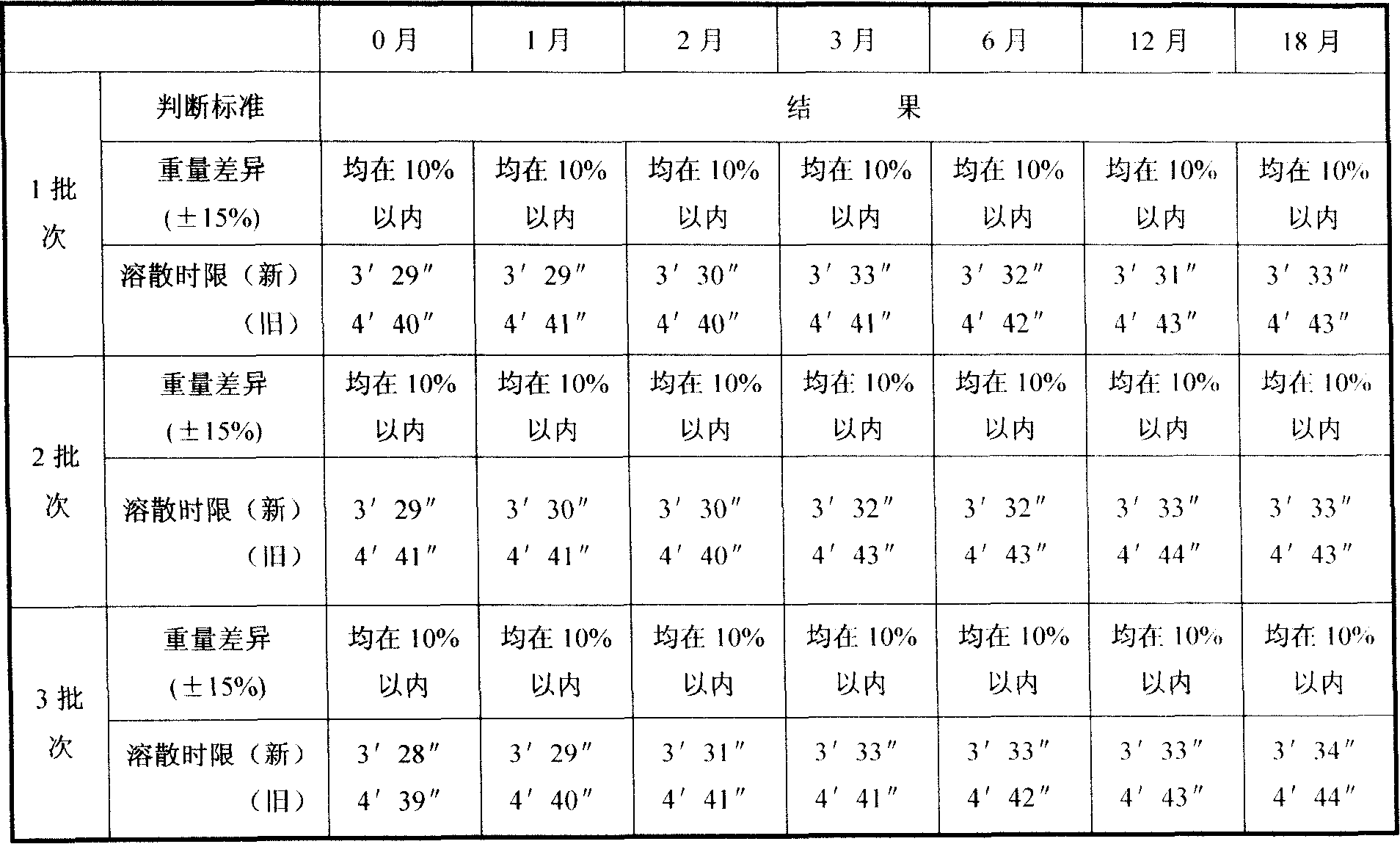Dropping pills containing ursolic acid and method for preparing the same
A technology of ursolic acid and dropping pills, applied in the field of medicine, can solve the problems of low degree of pure naturalness, accelerated dissolution rate, and low degree of pureness of the excipients of dropping pills, so as to reduce the irritating smell of medicines, improve the oral pH, pure highly natural effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
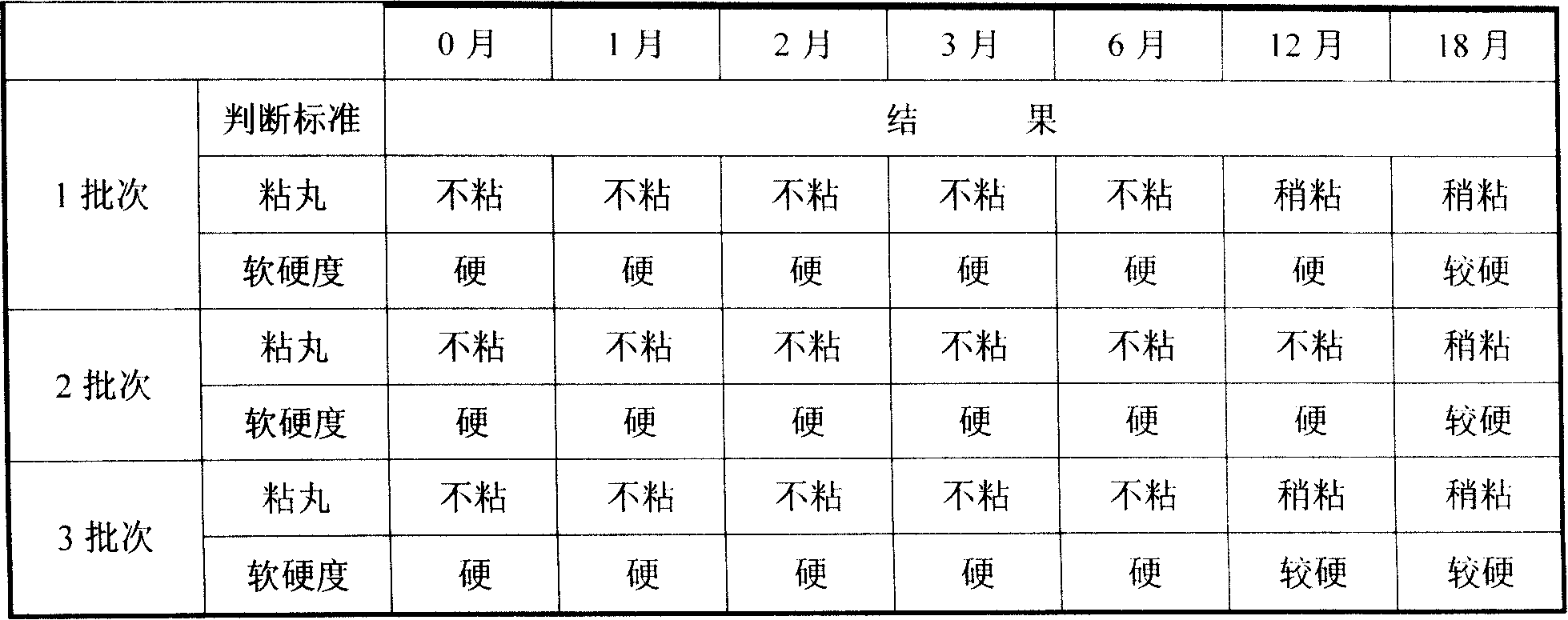
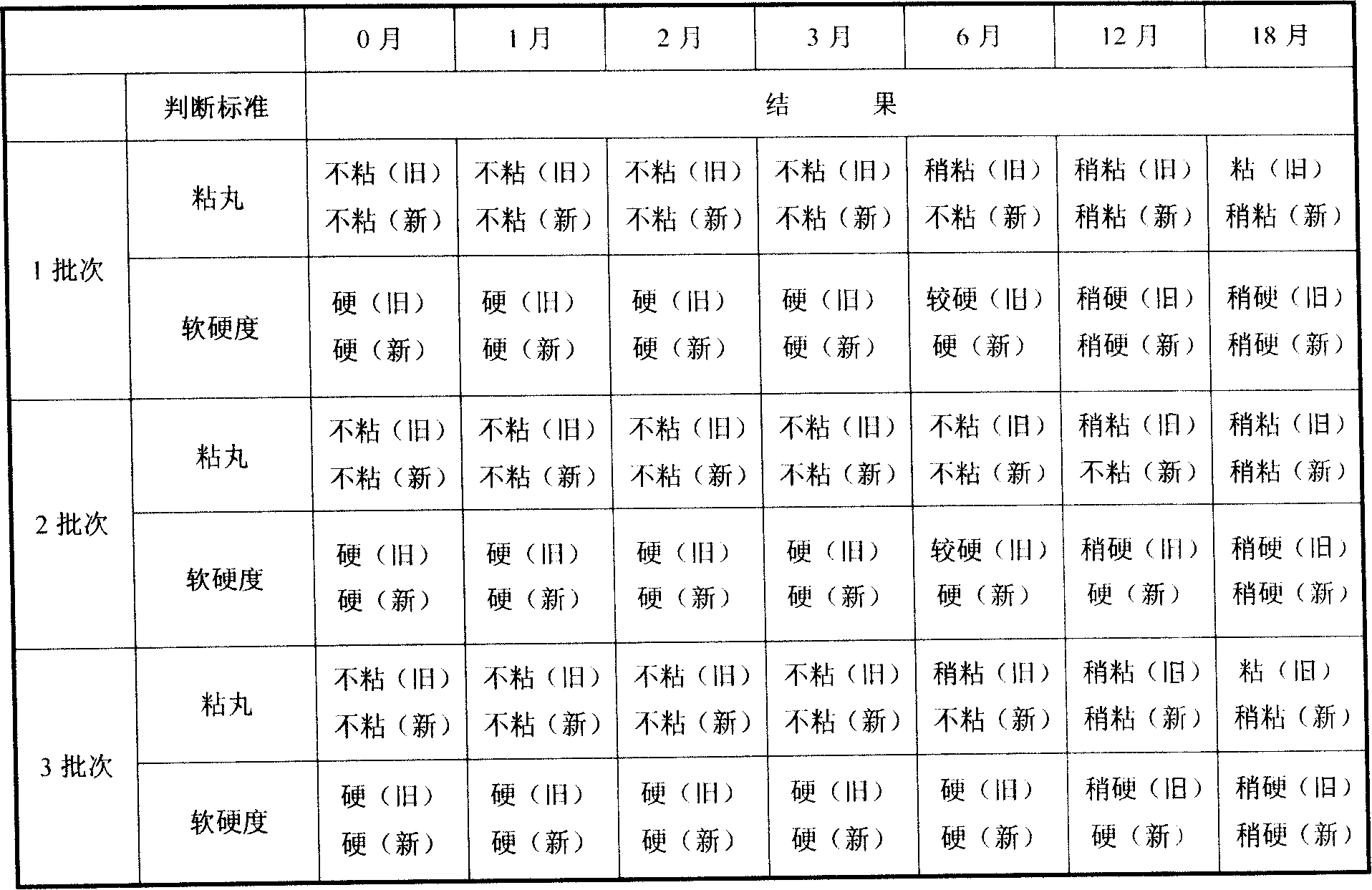
Embodiment 1
[0054] (a) get ursolic acid fine powder 6.0g, xylitol 18.3g, starch 6.7g for subsequent use;
[0055] (b) Mix xylitol and starch evenly, add the above-mentioned ursolic acid fine powder and mix well, heat and melt the mixture at 64°C, stir evenly, keep stirring for 25 minutes, keep warm, drip at 64°C, dropper caliber 1.2 to 2.5 mm in diameter, drop into methyl silicone oil at 24°C to make 1000 drop pills, drain the drop pills and wipe off the cooling liquid.
Embodiment 2
[0057] (a) Get ursolic acid fine powder 20g, lactitol 76.9g, starch 23.1g for subsequent use;
[0058] (b) Take lactitol and starch and mix evenly, add the above-mentioned ursolic acid fine powder and mix well, the mixture is heated and melted at 64°C, stirred evenly, the stirring time is 20 minutes, kept warm, dripped at 64°C, the diameter of the dropper is 1.2 to 2.5 mm, drop into methyl silicone oil at 28°C to make 3,000 drop pills, drain the drop pills and wipe off the cooling liquid.
Embodiment 3
[0060] (a) get ursolic acid fine powder 40g, xylitol 71.4g, gum arabic 28.6g for subsequent use;
[0061] (b) Mix xylitol and gum arabic evenly, add the above-mentioned ursolic acid fine powder and mix well, heat and melt the mixture at 64°C, stir evenly, keep stirring for 10 minutes, keep warm, drip at 64°C, dropper The caliber is 1.2-2.5 mm, drop into methyl silicone oil at 25°C to make 4,000 drop pills, drain the drop pills and wipe off the cooling liquid, and the product is ready.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Caliber | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com