High-endophilicity targeting amalgamation protein
A fusion protein and genetic engineering technology, applied in the field of high-affinity targeted fusion proteins, can solve the problems of strong non-specific toxicity, low affinity, and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] According to the principle of codon preference in Escherichia coli, the gene sequence of SEQ ID NO: 1 was synthesized by the method of total gene synthesis combined with PCR, and then SEQ ID NO: 2-8 and the currently known GnRH- PE derivatives, gene sequences of three control test fusion proteins GnRH-PE40 (SEQ ID NO: 9), (Ala6) GnRH-PE40 (SEQ ID NO: 10) and (Ala6) GnRH-PE40KDEL (SEQ ID NO: 11) . After sequencing verification, connect the target gene into the expression vector, transfer the verified recombinant plasmid into the host bacteria, obtain the target bacteria after expression and fermentation, and then break the bacteria by osmotic pressure method, centrifuge to take the supernatant, pass The methods of hydrophobic chromatography and ion exchange chromatography were purified to obtain 8 targeted fusion proteins of the present invention with a purity greater than 95% and three kinds of control test fusion proteins GnRH-PE40, (Ala6) GnRH-PE40 and (Ala6) GnRH- P...
example 2
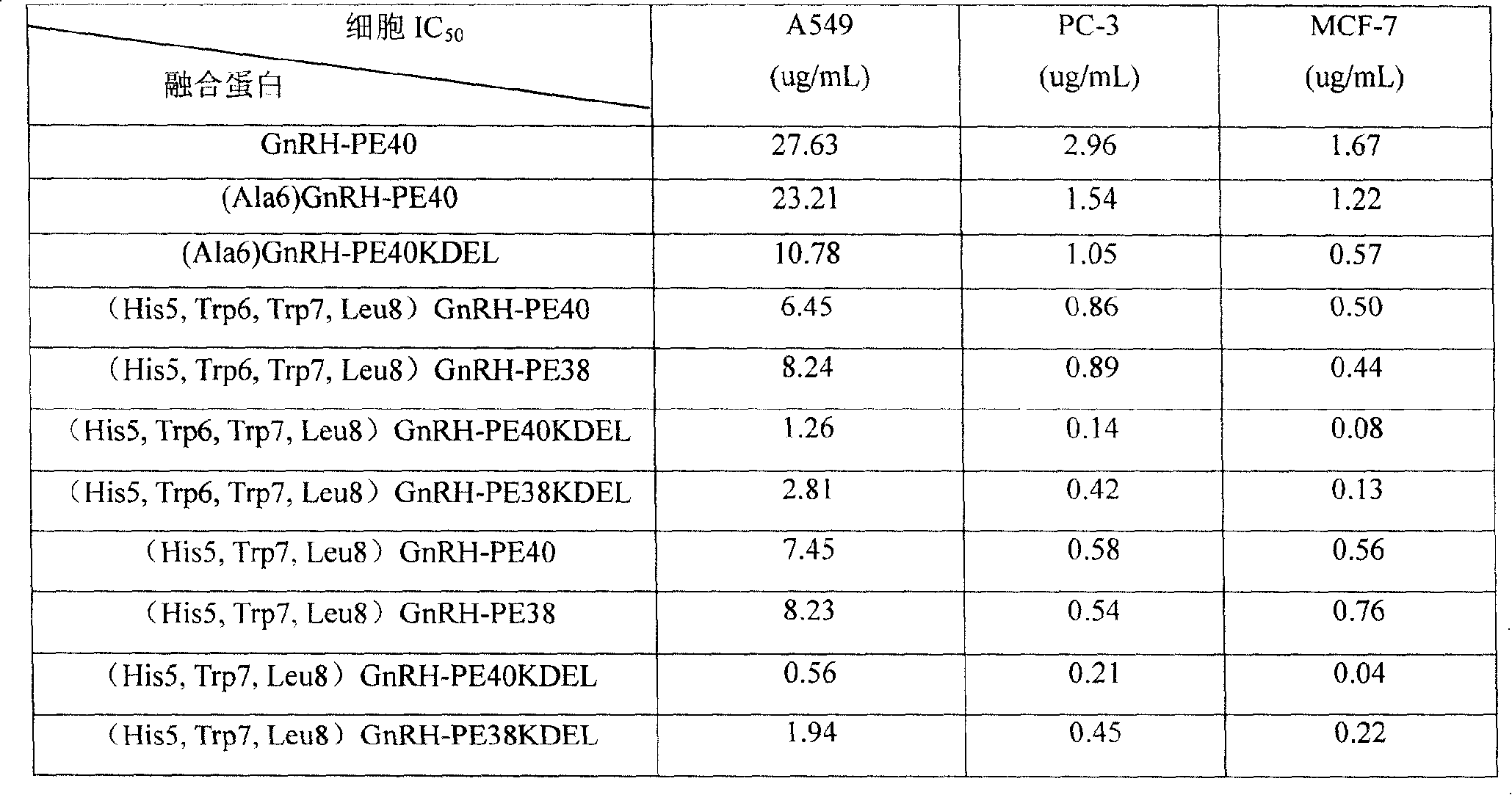
[0043] Take eight kinds of targeted fusion proteins in the present invention and three kinds of control fusion proteins to carry out cell activity test: use MTT method, first digest and collect Hela cells, and dilute the cells to 6-8×10 with RPMI 1640 culture medium 4 cell suspension per milliliter, seeded in 96-well plate, 100ul / well, cultured at 37°C for 4 hours. Add 100ul culture solution to the control. The 11 kinds of samples were first diluted with RPMI 1640 culture medium to 100ul / mL as the initial well, and then the samples were diluted according to the relationship of 2.5 times between each well and the upper well, and each diluted sample was added to each well with 100ul, 37 degrees, and incubated for 24 hours. Take it out, stain it with MTT method, measure it with a microplate reader at 570nm and calculate the IC50 value. The results of the activity assay are shown in the table below,
[0044]
example 3
[0046] Get eight kinds of targeted fusion proteins in the present invention and three kinds of control test fusion proteins to carry out affinity test (affinity measurement of target protein and GnRH receptor): prepare placental plasma membrane, get placental villi, place in ice-cold 25mM PB, pH7.4, 1mM MgCl 2 Homogenize in medium, filter, and centrifuge at 10000g for 20min, remove the supernatant, wash the precipitate once with the above buffer, then centrifuge at 10000g for 15min, suspend the precipitate in the above buffer at a wet weight ratio of 60mg / mL for later use, and use the Lowry method protein concentration. Iodine labeling of targeted fusion proteins: add 3.8 x 10 to a 1.5 mL doff tube 7 Bq Na 125 I, 20uL 0.5M PB, pH7.5, 500ug targeting fusion protein, 2.3mU lactate catalase β-D(+)-glucose to start the reaction, react at 20°C for 10min, then add 50uL 0.1M boric acid buffer pH9. 2 Terminate the reaction. Chromatographic purification was then performed using 1 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com