Molecules and chimeric molecules thereof
A technology of chimeric molecules and proteins, applied in growth factors/inducing factors, drug combinations, animal/human proteins, etc., can solve problems such as pollution, inapplicability to clinical applications, and harmful transplantation applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
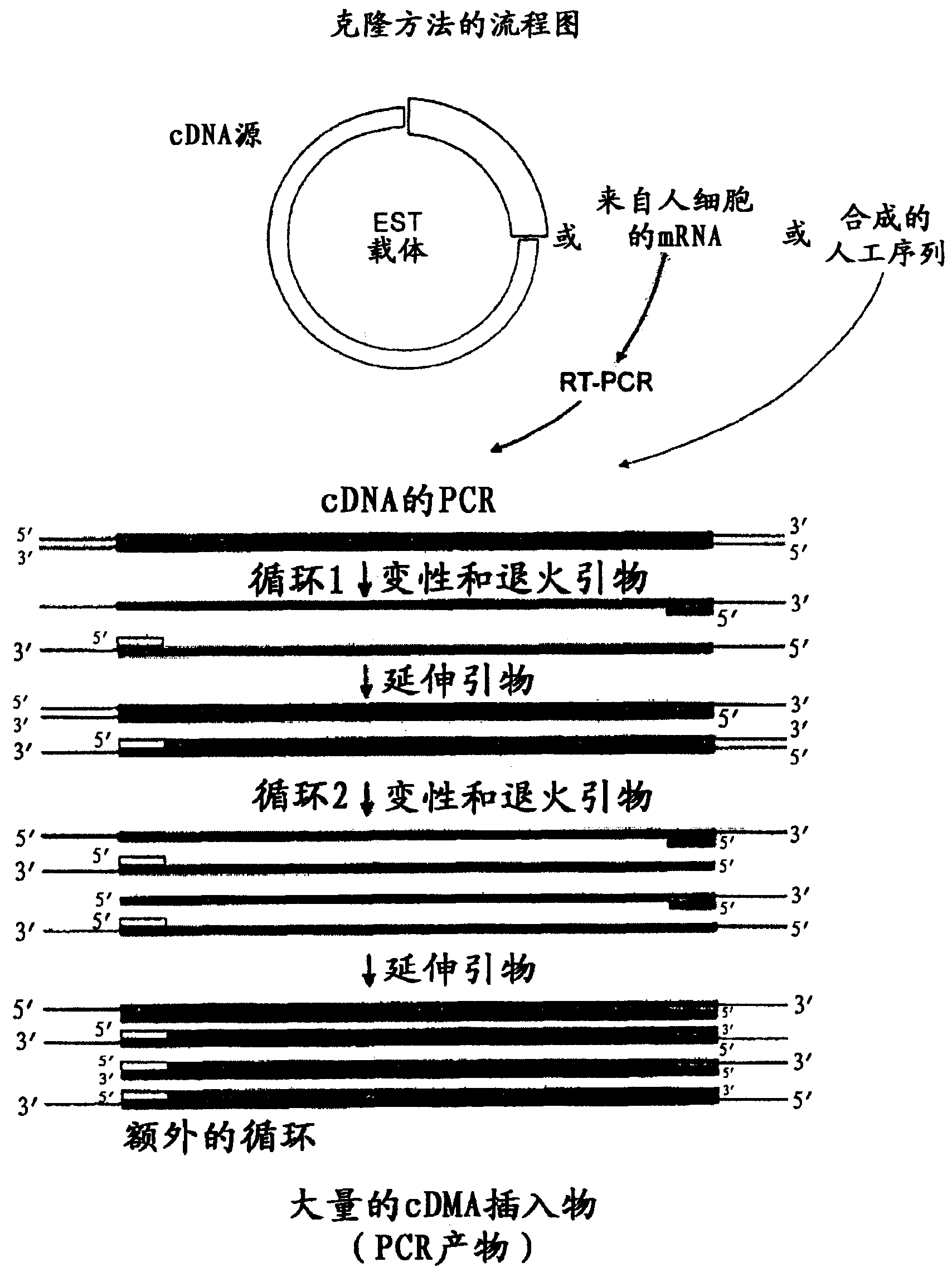
[1055] Cloning of proteins of the invention
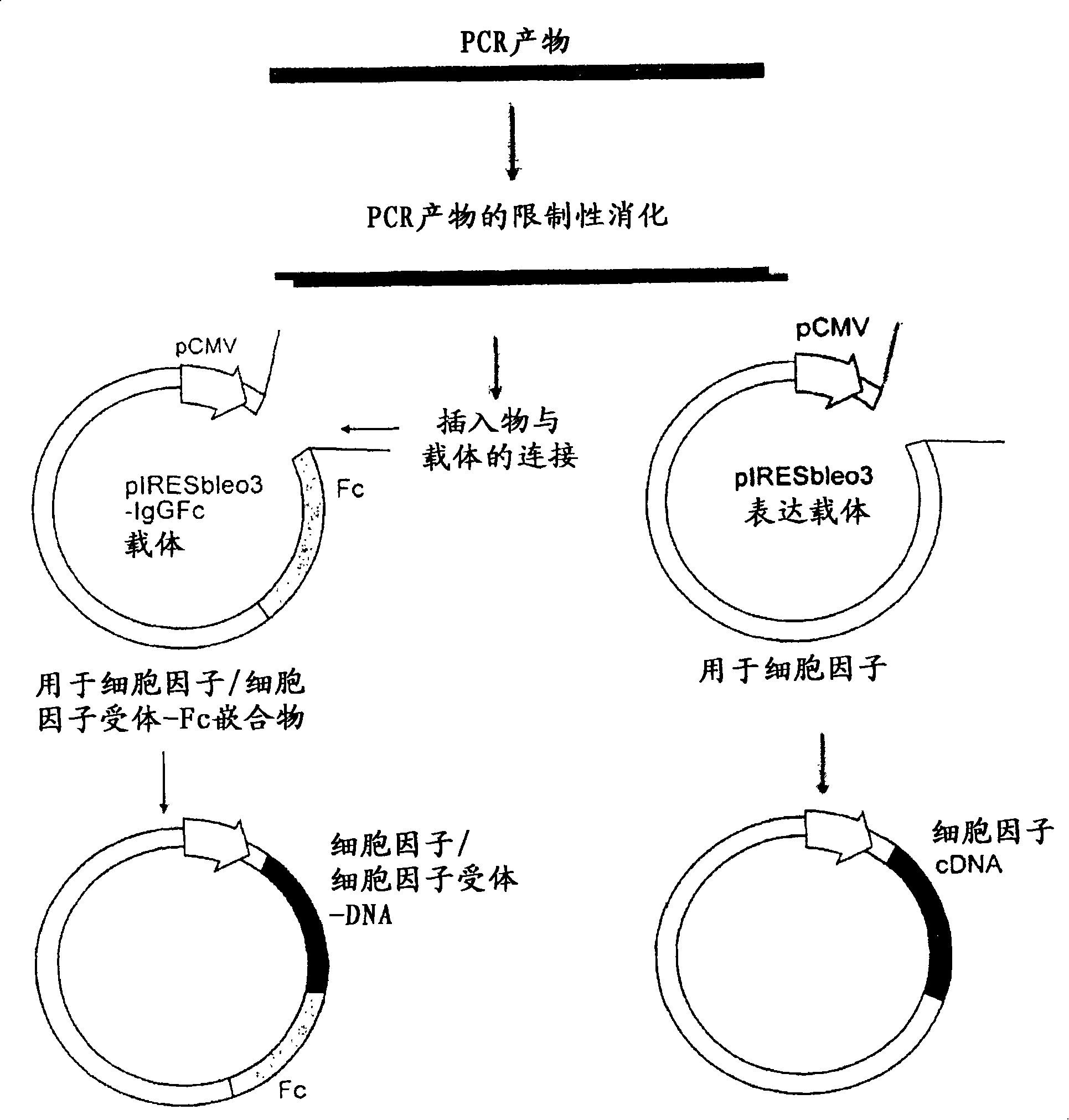
[1056] (a) Preparation of pIRESbleo3-Fc construct
[1057] The DNA sequence encoding the Fc domain of human IgG1 was amplified by polymerase chain reaction (PCR) from an EST cDNA library (Clone ID 6277773, Invitrogen) using cDNAs incorporating restriction enzymes BamH1 and BstX1 sites, respectively. Forward primer (SEQ ID NO: 21) and reverse primer (SEQ ID NO: 22). This amplicon was cloned into the corresponding restriction site in pIRESbleo3 (Cat. No. 6989-1, BD Biosciences) to make construct pIRESbleo3-Fc. Digestion of pIRESbleo3-Fc with BamH1 and BstX1 released an insert of the expected size of 780 bp as determined by gel electrophoresis.
[1058] (b) Preparation of DNA constructs expressing protein or protein-Fc
[1059] The DNA sequence encoding the protein or its extracellular domain was amplified by PCR from the EST cDNA library using forward and reverse primers with restriction enzyme sites introduced according to Table ...
Embodiment 2
[1067] (a) Preparation and purification of EPO of the present invention
[1068] (i) Preparation of EPO of the present invention
[1069] On day 0, use 3 × 10 cells of the transformed embryonic human kidney cell line 7 Cell seeding five 500cm 2 Tissue culture dishes (Corning) with cells such as HEK 293, HEK 293c18, HEK 293T, 293CEN4, HEK 293F, HEK 293FT, HEK 293E, AD-293 (Stratagene) 293A (Invitrogen). Cells were inoculated into 90ml of Dulbecco's modified Eagle's medium / Ham's nutrient mixture F12 (DMEM / F12) (JRH Biosciences) per plate, and the medium was supplemented with 10% (v / v) heat-inactivated fetal calf serum (FCS , JRH Biosciences), 10 mM HEPES (Sigma), 4 mM L-glutamine (Ameresco) and 1% (v / v) penicillin-streptomycin (JRH Biosciences).
[1070] On the first day, transfection was performed with calcium phosphate. Before transfection, the medium in each plate was replaced with 120 ml of fresh DMEM / F12. Prepare a calcium phosphate / DNA precipitate by adding 1200 μg of...
Embodiment 3
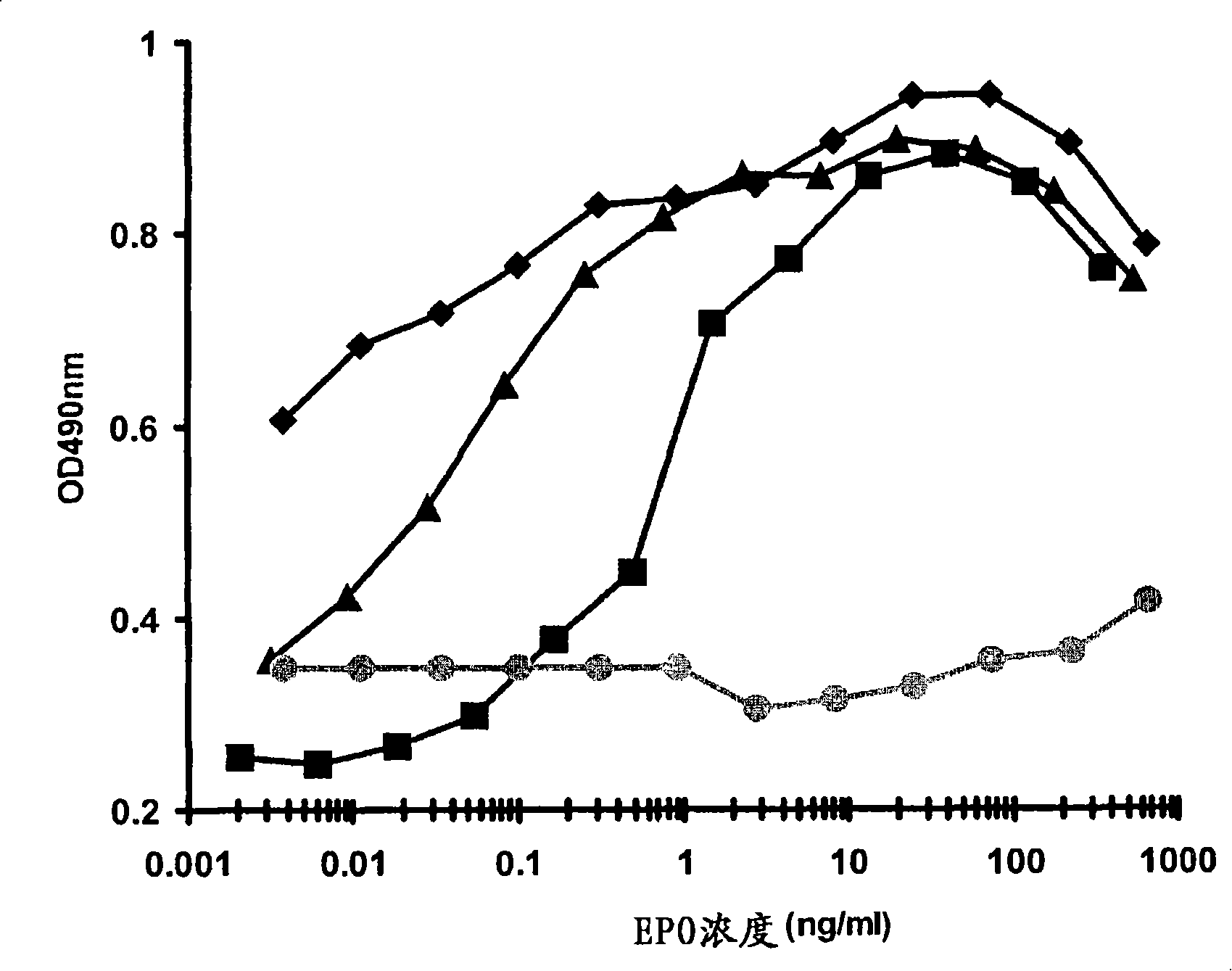
[1145] (a) Features of EPO of the present invention
[1146] (i) Two-dimensional polyacrylamide electrophoresis
[1147] Samples collected from Example 2(a) were buffer exchanged through a dialysis or desalting column (Pharmacia HR 10 / 10 Fast Desalting Column) into repurified (18 MOhm) water and dried with a SpeedVac concentrator. Then, the sample was redissolved in 240 μl MSD buffer (5M urea, 2M thiourea, 65mM DTT, 2% (w / v) CHAPS, 2% (w / v) thiobetaine 3-10, 0.2% (v / v) vehicle ampholyte, 40 mM Tris, 0.002% (w / v) bromophenol blue, water) and centrifuged at 15000 g for 8 minutes.
[1148] Isoelectric focusing (IEF) was performed with precast 11 cm or precast 17 cm gels pH 3-10 immobilized pH gradient IEF strips (BioRad). IEF strips were rehydrated with samples in closed tubes at room temperature for at least 6 hours. The IEF strips were placed in the focusing chamber and covered with liquid paraffin. IEF was performed at 100V for 1 hour, 200V for 1 hour, 600V for 2 hours, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
| Apparent molecular weight | aaaaa | aaaaa |
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com