Coordination polymer type ion exchange material, preparation and application thereof
An ion exchange material, coordination polymer technology, applied in the direction of ion exchange, anion exchange, organic anion exchanger, etc., can solve the problems of difficult ion exchange process, difficult post-processing, changes, etc., to achieve high yield, reaction The effect of short time and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
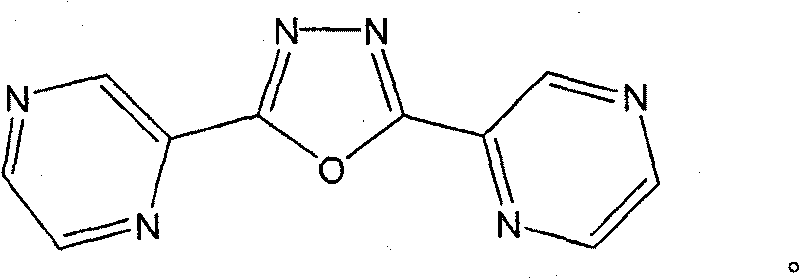
[0030] Preparation and Discussion of Bispyrazine-Bridged Organic Ligand L Containing Oxadiazole Ring
[0031] 1. Synthesis experiment and post-processing:
[0032] 18.6 g of pyrazine-2-carboxylic acid (0.15 mol) and 12.8 g of hydrazine hydrochloride hydrate (0.08 mol) were placed in a three-necked flask and stirred and mixed, and then 50 mL of phosphoric acid (85%) was added. Subsequently, 20.1 g of phosphorus pentoxide (0.48 mol) and 23.0 g of phosphorus oxychloride (0.15 mol) were added successively. The viscous solution was heated to 140°C and stirred vigorously for four hours, then cooled to 17°C. Pour twice distilled water into the obtained mucus until the mucus and water are completely combined, and after dissolving, neutralize with sodium bicarbonate until the pH value of the solution is 7. An orange-yellow precipitate precipitated, was filtered, washed three times with 60 mL of anhydrous ether, and then dried in vacuo. Accurately weighed with an analytical balance, ...
Embodiment 2
[0042] Preparation and Discussion of Bispyrazine-Bridged Organic Ligand L Containing Oxadiazole Ring
[0043] 1. Synthesis experiment and post-processing:
[0044] 4.65 g of pyrazine-2-carboxylic acid (0.0375 mol) and 3.2 g of hydrazine hydrochloride (0.02 mol) were placed in a three-necked flask and stirred and mixed, and then 15 mL of phosphoric acid (85%) was added. Subsequently, 5.03 g of phosphorus pentoxide (0.12 mol) and 5.8 g of phosphorus oxychloride (0.0375 mol) were carefully added in succession. The viscous solution was heated to 140°C and stirred vigorously for four hours, then cooled to 25°C.
[0045] Post-treatment process: Pour twice distilled water into the obtained mucus until the mucus and water are completely fused, dissolve and neutralize with sodium bicarbonate until the pH of the solution is 7. An orange-yellow precipitate precipitated, was filtered, washed three times with 20 mL of anhydrous ether, and then dried in vacuo. Weigh accurately with an an...
Embodiment 3
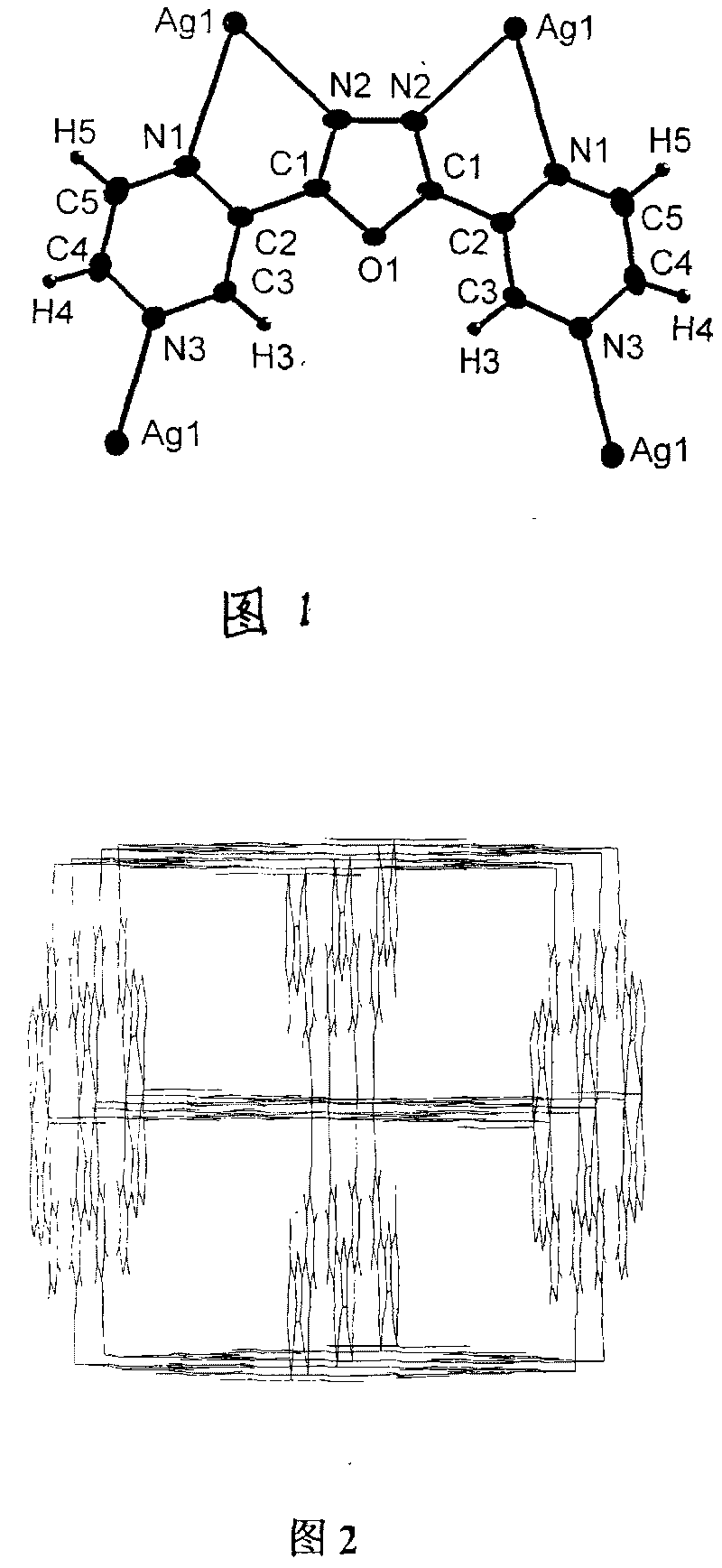
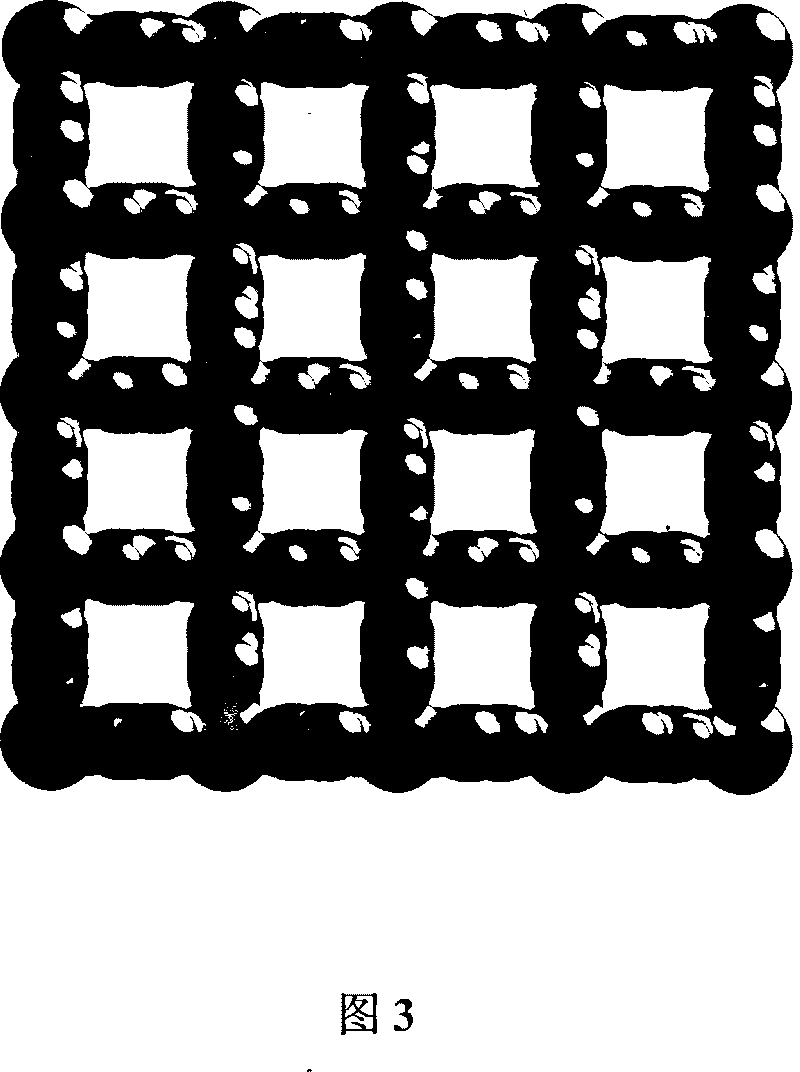
[0048] Preparation, Structure and Discussion of Results of Coordination Polymer Ion Exchange Materials
[0049] 1. Preparation of new materials:
[0050] Anion is BF 4 - Preparation of ion-exchange materials: AgBF at 30 °C 4 (2mmol, 0.39g) and an equimolar amount of L ligand (2mmol, 0.45g) were dissolved in methanol / chloroform (commercially available analytical grade, volume ratio 1:1, 60mL) organic solvent, stirred for 1 hour, and then the obtained The clear solution was left to stand, and with the volatilization of the solvent, the ion exchange material could be obtained after 4 days. Filter, wash with 40 mL of anhydrous ether, and finally vacuum-dry. Calculated yield: 80%.
[0051] Anion is AsF 6 - Preparation of coordination polymer ion exchange materials: AgAsF at 30 °C 6 (2mmol, 0.60g) and an equimolar amount of L ligand (2mmol, 0.45g) were dissolved in methanol / chloroform (commercially available analytical grade, volume ratio 1:1, 80mL) organic solvent, stirred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com