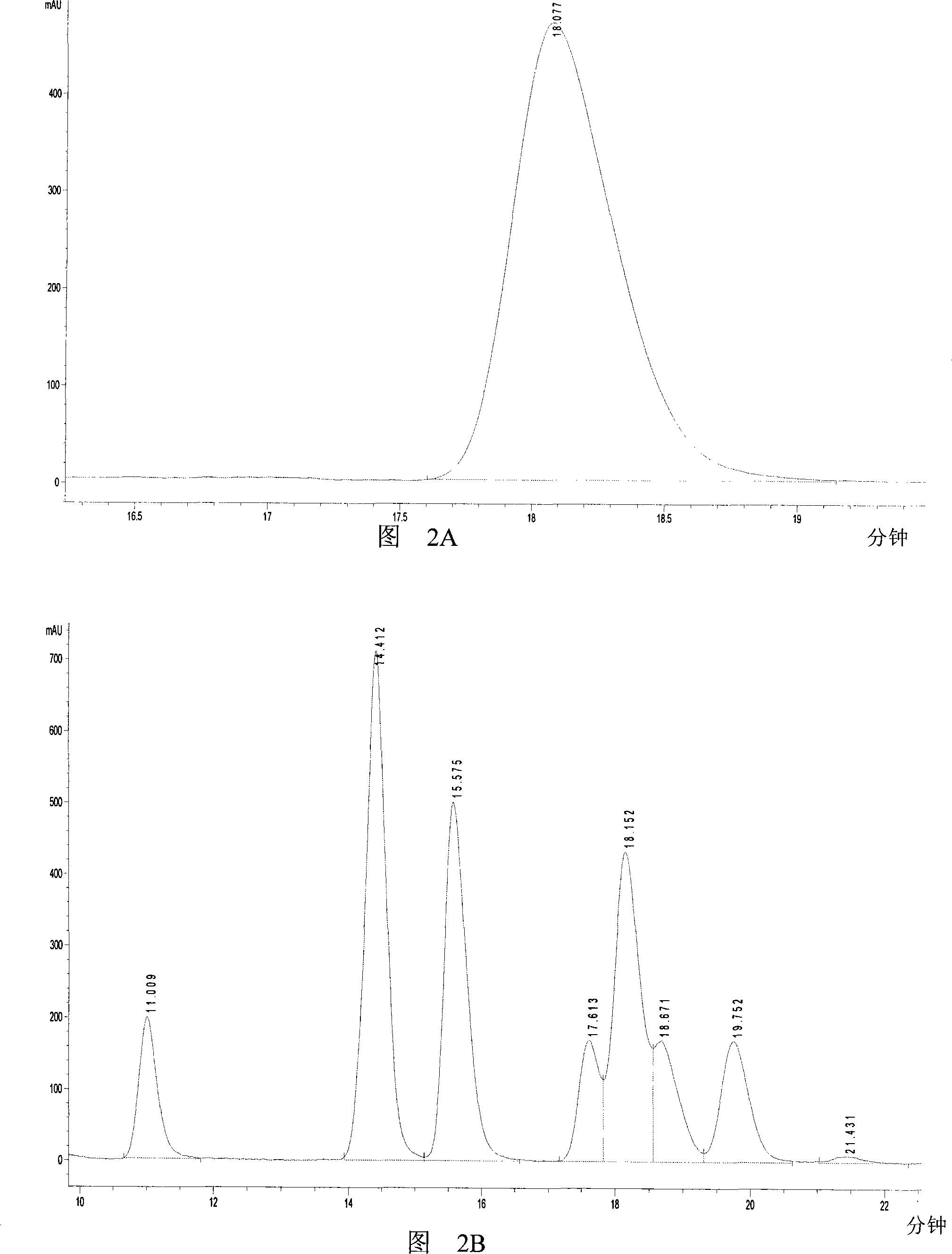Method for synthesizing lactic acid by using glycerol
A technology of glycerin and lactic acid, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as equipment corrosion, high equipment requirements, harsh production equipment, etc. The effect of simple system and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, glycerin oxidation directly synthesizes lactic acid
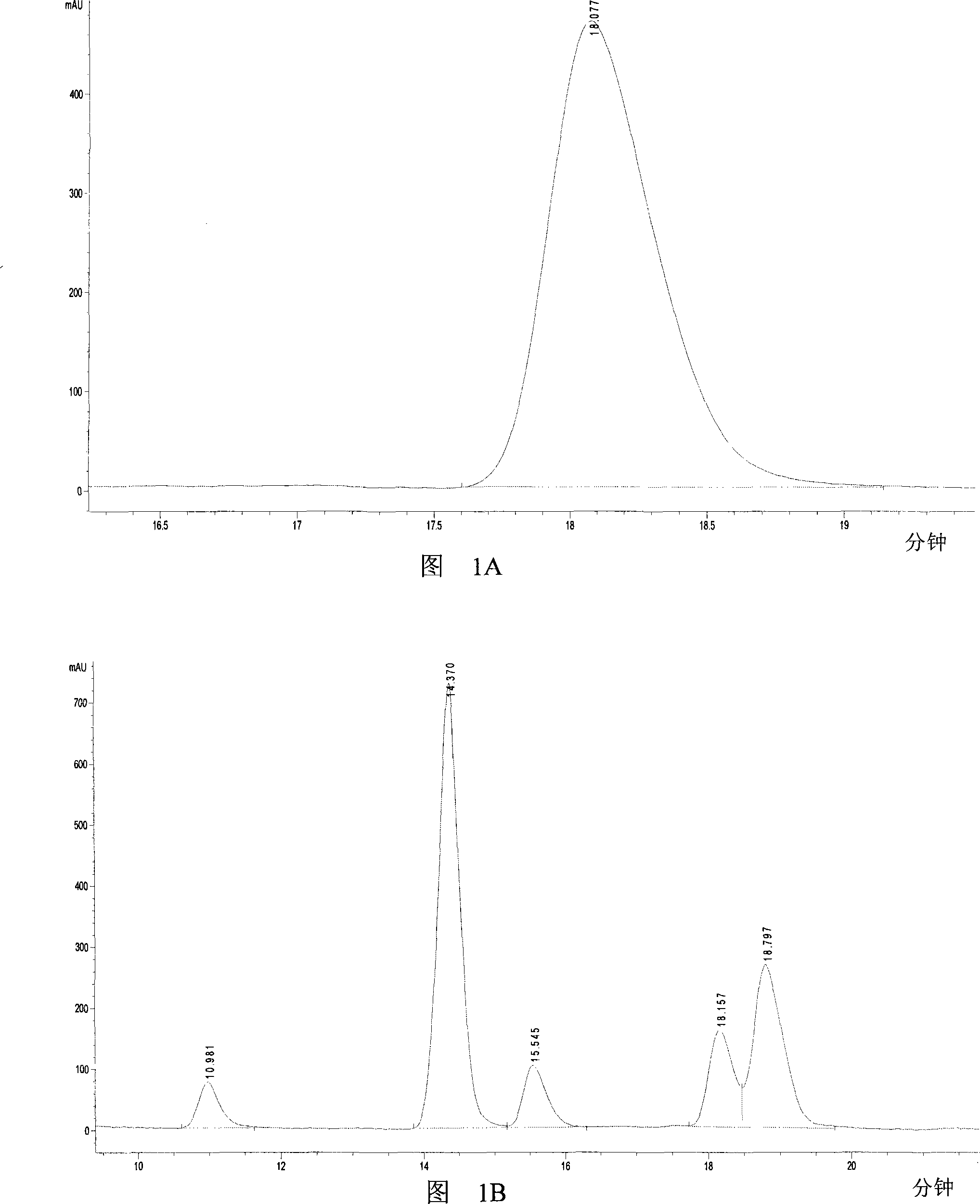
[0034] 2 g of glycerol (Glycerol A.R. Beijing Chemical Reagent Company) and 3.46 g of sodium hydroxide (Sodium hydroxide A.R. Beijing Beihua Fine Chemicals Co., Ltd.) were dissolved in 40 mL of water and transferred to a 100 mL three-neck round bottom flask. Add 0.13g Au / TiO 2 Catalyst (World Gold Council World Gold Council), the O 2 , heated to 90°C, and reacted for 3 hours. The experiment was repeated three times, each with 10 bottles.
[0035] After the reaction finishes, measure the amount of lactic acid in the reaction solution according to the following method:
[0036] The reaction products were qualitatively and quantitatively analyzed by high performance liquid chromatography (Agilent 1100 Series HPLC, Beijing Agilent Co., Ltd., the separation column was: Alltech OA-1000 Organic Acids, diluted with H 2 SO 4 The aqueous solution is the mobile phase, the flow rate is 0.3ml / min, the ultraviol...
Embodiment 2
[0043] Embodiment 2, glycerol oxidation direct synthesis lactic acid
[0044] 2 g of glycerol (Glycerol A.R. Beijing Chemical Reagent Company) and 1.73 g of sodium hydroxide (Sodiumhydroxide A.R. Beijing Beihua Fine Chemical Co., Ltd.) were dissolved in 40 mL of water and transferred to a 100 mL three-neck round bottom flask. Add 0.10g Au / C catalyst (World Gold Council World Gold Council), feed 1atm flow rate and be the O of 70ml / min 2 , heated to 140°C, and reacted for 2 hours. The experiment was repeated three times, each with 10 bottles.
[0045] After the reaction, according to the method described in Example 1, the amount of lactic acid in the reaction solution was measured.
[0046] The chromatographic detection results of the reaction products are shown in Figure 2. Lactic acid standard curve is y=277504x-13.315, R 2 = 0.9999. The result shows that the retention time of the peak of the product lactic acid on the chromatogram is exactly the same as that of the lacti...
Embodiment 3
[0048] Embodiment 3, glycerol oxidation directly synthesizes lactic acid
[0049] 0.8 g of glycerol (Glycerol A.R. Beijing Chemical Reagent Company) and 3.46 g of potassium hydroxide (Potassium hydroxide A.R. Beijing Beihua Fine Chemical Co., Ltd.) were dissolved in 40 mL of water and transferred to a 100 mL three-neck round bottom flask. Add 0.13g Au / ZrO 2 Catalyst catalyst (World Gold Council World Gold Council), the O 2 , heated to 60°C, and reacted for 4 hours. The experiment was repeated three times, each with 10 bottles.
[0050] After the reaction, according to the method described in Example 1, the amount of lactic acid in the reaction solution was measured.
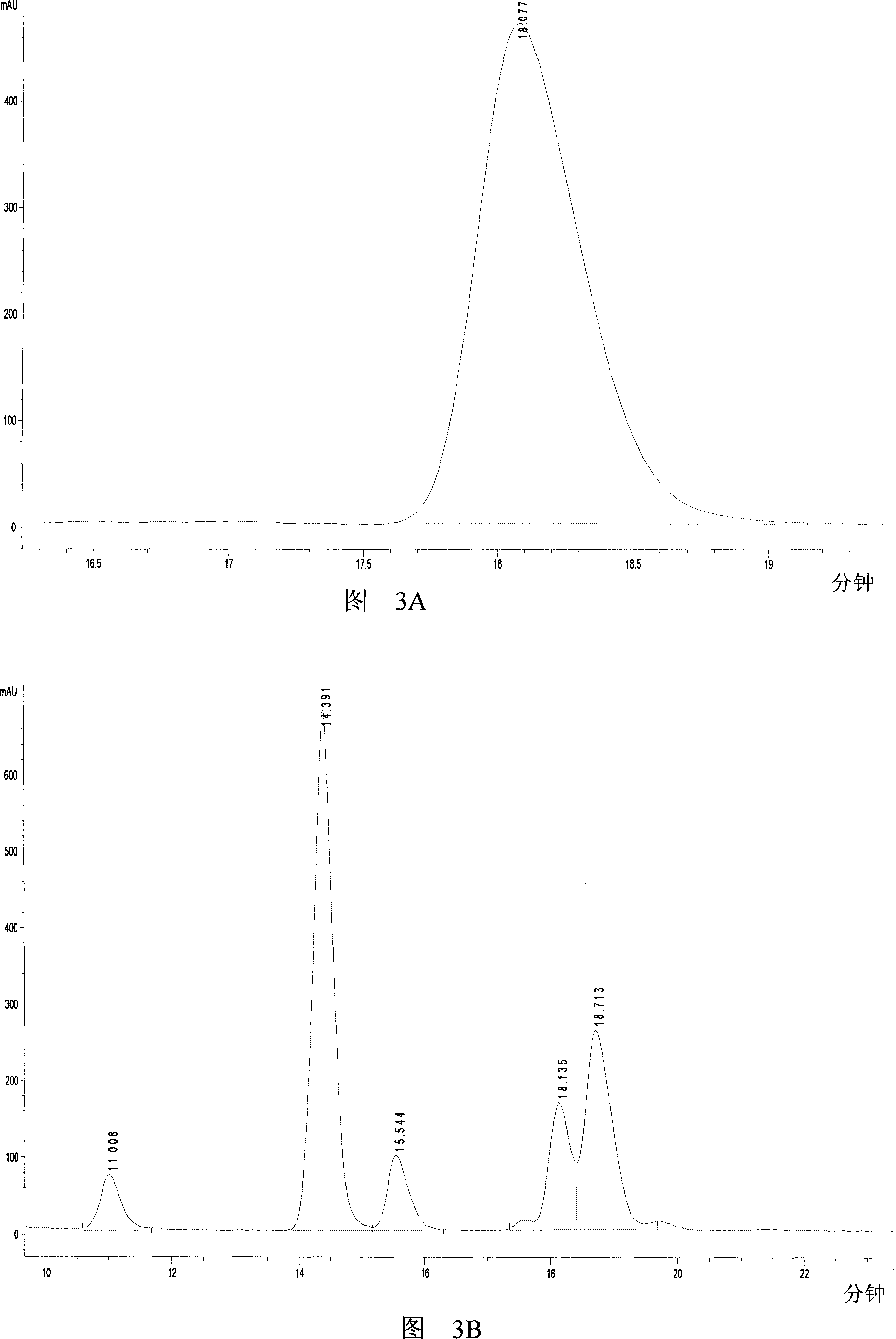
[0051] The chromatographic detection results of the reaction products are shown in Figure 3. Lactic acid standard curve is y=277504x-13.315R 2 = 0.9999. The result shows that the retention time of the peak of the product lactic acid on the chromatogram is exactly the same as that of the lactic acid standard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com