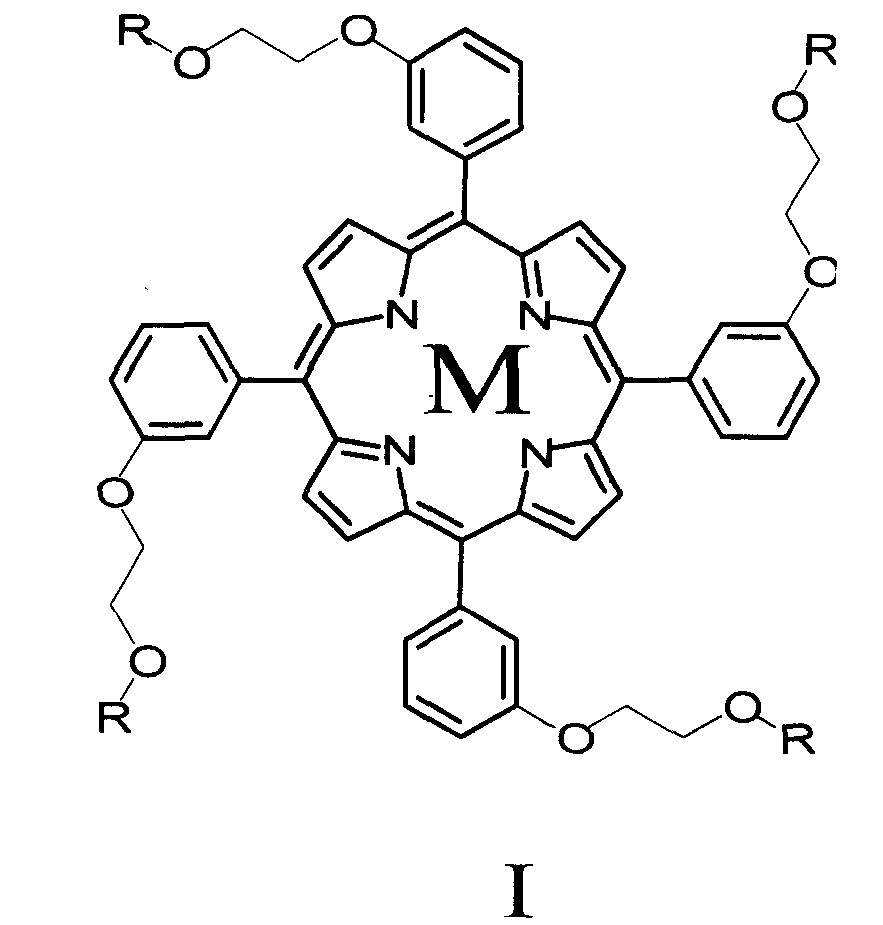
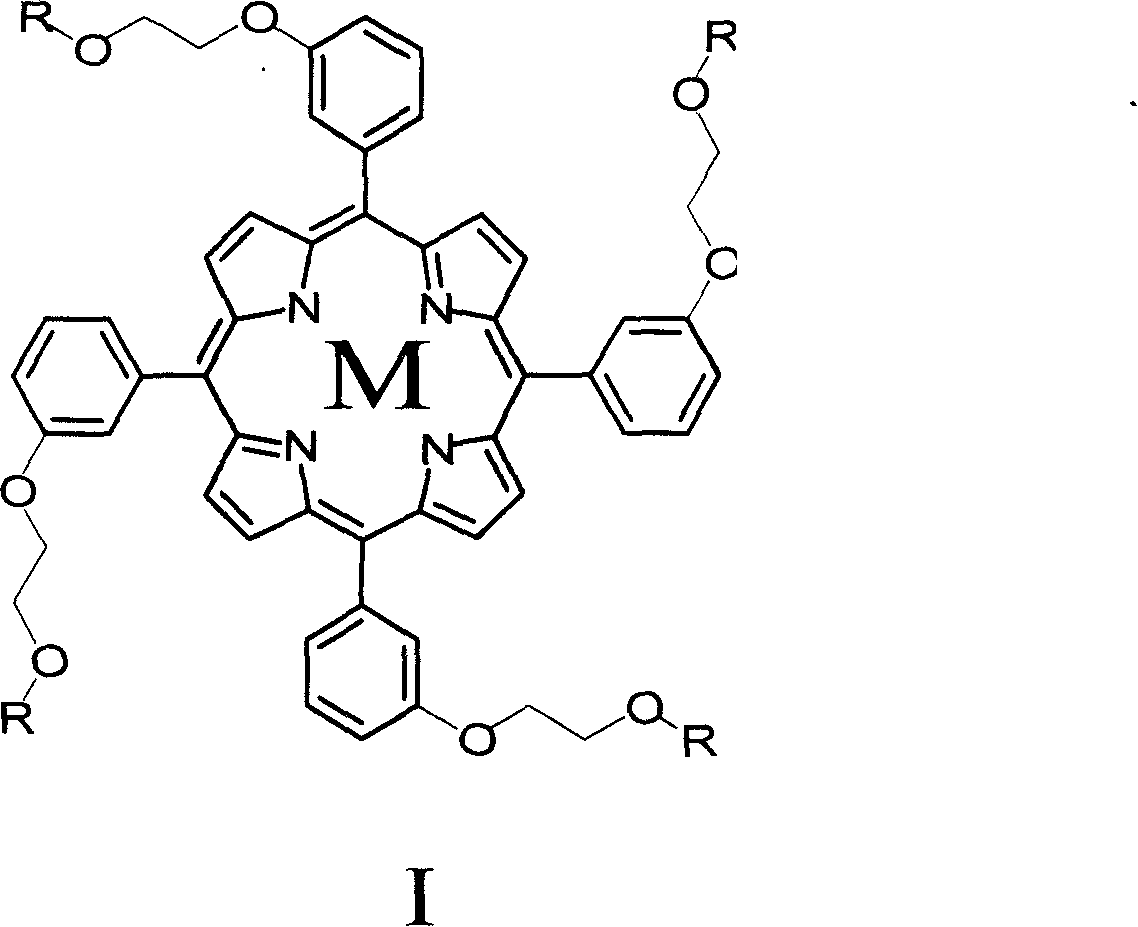
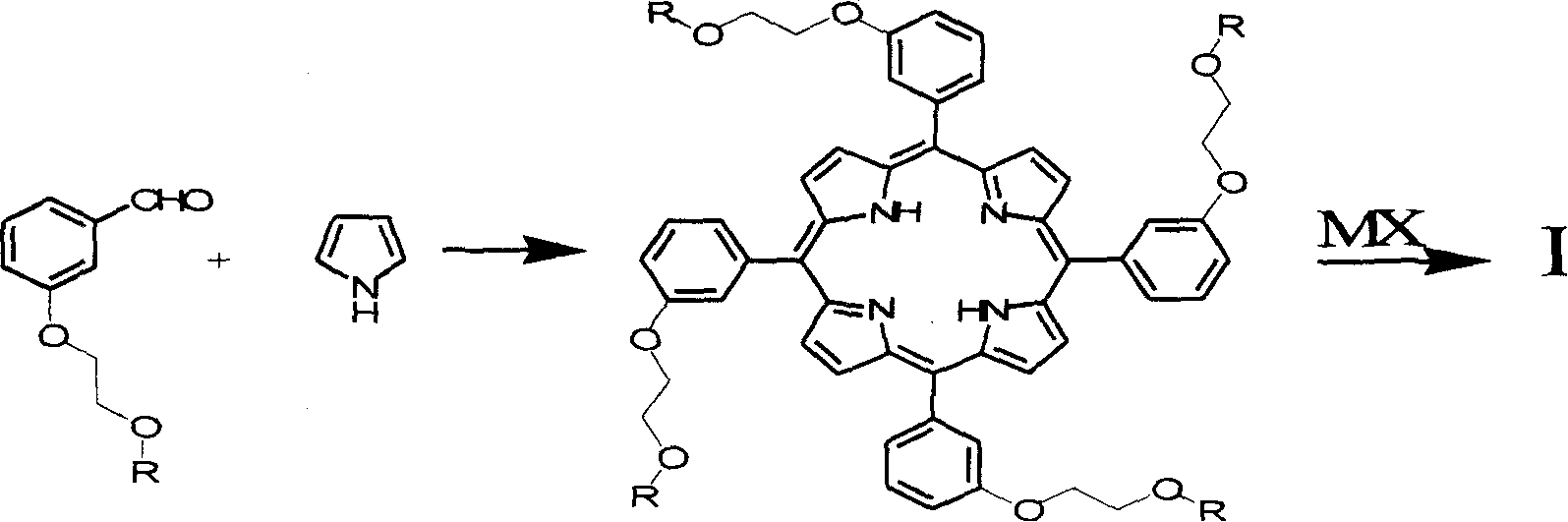
Porphyrin derivative and application for the same as small molecule antioxidant
A derivative, porphyrin technology, applied in a class of porphyrin derivatives and its application as a small molecule antioxidant, can solve the problems of limiting drug feasibility, high cost, and inability to enter cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of 3-(2-methoxyethoxy) benzaldehyde
[0034]
[0035] Dissolve 100 grams of m-hydroxybenzaldehyde in 1 liter of DMF, add 226 grams of anhydrous potassium carbonate, stir for 30 minutes, add 81.3 grams of 2-chloroethyl methyl ether, heat and reflux for 6 hours, evaporate DMF under reduced pressure, add Dissolve in 500 ml of water, filter, extract the filtrate 3 times with dichloromethane, combine the extracts, wash with 200 ml of 2% sodium hydroxide solution, wash with water 3 times, dry over anhydrous sodium sulfate, filter, evaporate the solvent, 154 g of product were obtained. ESI-MS: [M+H] + , 181; 1 H NMR (DMSO-d 6 ): δ3.31(s, 3H), 3.67(t, J=4.56Hz, 2H), 4.17(t, J=4.56Hz, 2H), 7.30(m, 1H), 7.43(m, 1H), 7.51 (m, 2H), 9.97(s, 1H).
Embodiment 2
[0036] Embodiment 2: the preparation of methoxyethyl p-toluenesulfonate
[0037]
[0038] Cool the mixture of 38 grams of ethylene glycol monomethyl ether and 120 grams of anhydrous pyridine to -6°C in an ice bath, add 101 grams of p-toluenesulfonyl chloride in batches, control the temperature not to exceed 0°C, and continue stirring for 30 minutes after the addition , remove the ice bath, and react at room temperature for 4 hours. The reaction mixture was slowly poured into a mixture of 250 ml of hydrochloric acid and 700 g of ice, stirred for 20 minutes, extracted 3 times with ethyl acetate, combined extracts, washed with water 3 times, dried over anhydrous sodium sulfate, and distilled off ethyl acetate to obtain 84 grams of product.
Embodiment 3
[0039] Embodiment 3: the preparation of 3-(2-methoxyethoxy) benzaldehyde
[0040]
[0041] Dissolve 12 grams of m-hydroxybenzaldehyde in 150 milliliters of DMF, add 27.6 grams of anhydrous potassium carbonate, stir, add 23 grams of methoxyethyl p-toluenesulfonate, heat and reflux for 3 hours, and evaporate DMF under reduced pressure , add 200 milliliters of water to dissolve, extract 3 times with dichloromethane, combine the extracts, wash with 200 milliliters of 5% sodium hydroxide solution, wash with water 3 times, dry over anhydrous sodium sulfate, filter, and distill off the solvent to obtain 16 grams of product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com