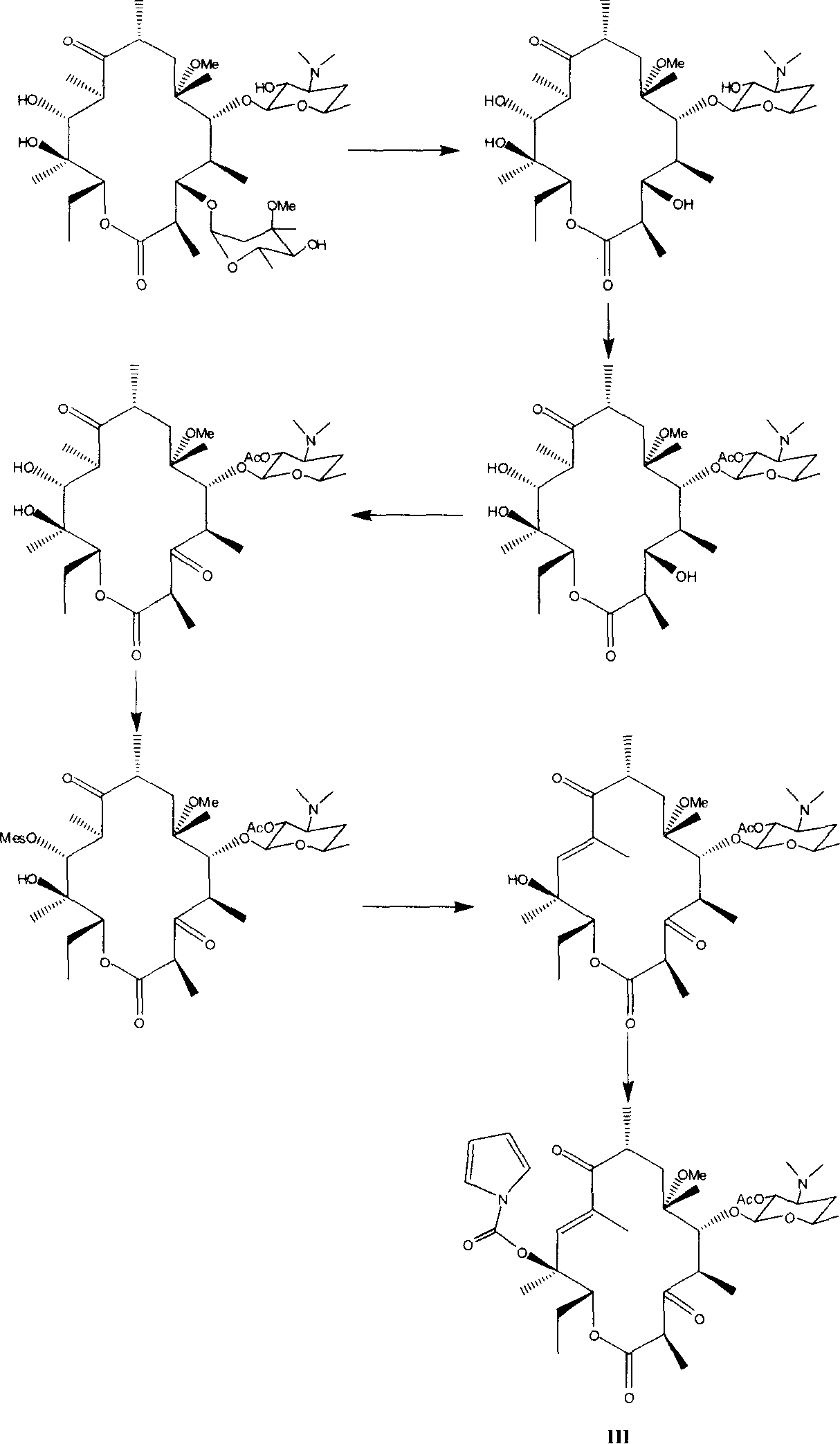
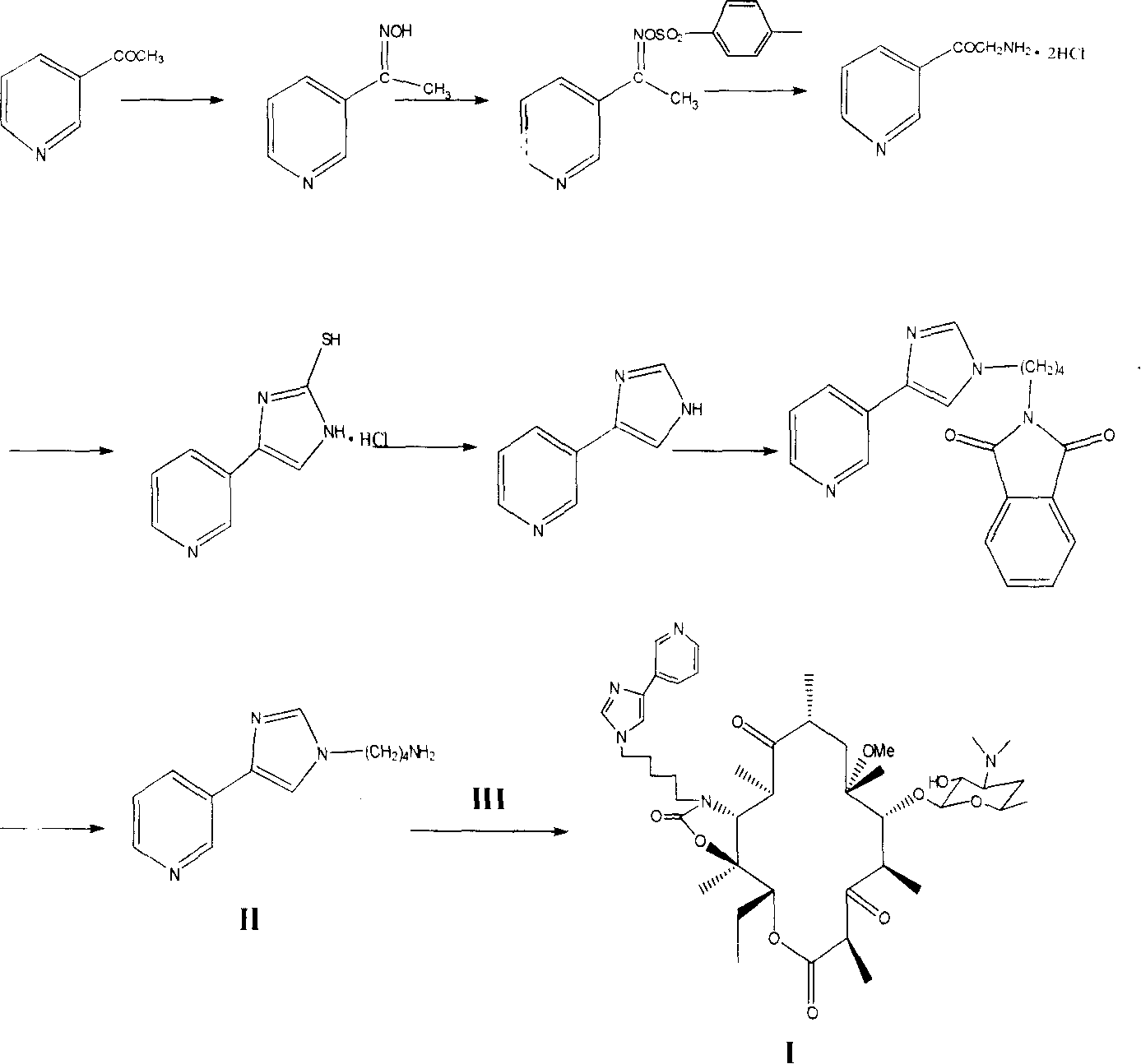
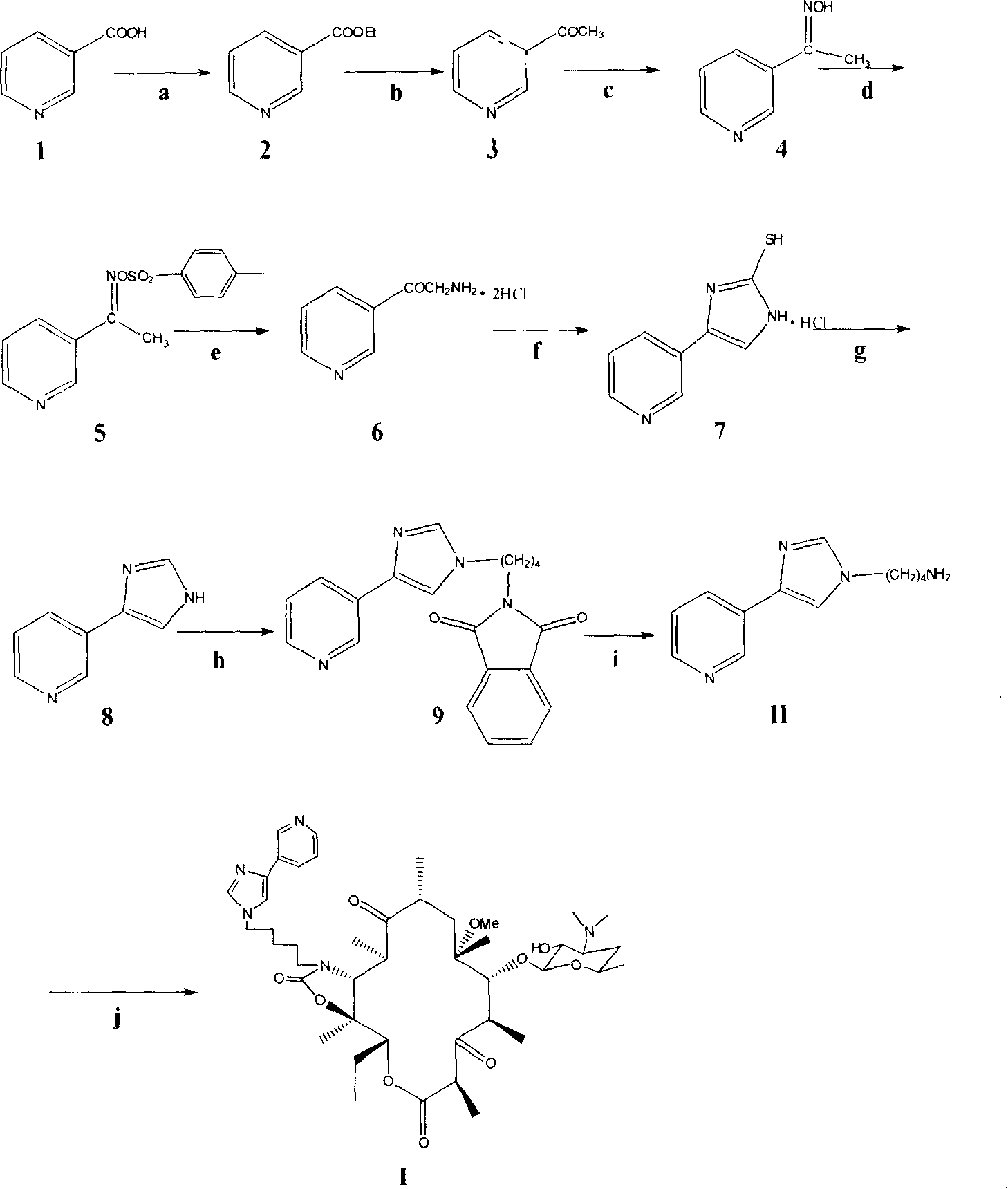
Semi-synthesis method for preparing antibiotic telithromycin
A technology of telithromycin and a synthesis method, which is applied in the directions of sugar derivatives, bulk chemical production, organic chemistry, etc., to achieve the effects of less by-products, easy separation, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 3-acetylpyridine oxime (4):
[0032] 10g (82.5mmol) of 3-acetylpyridine (3), methanol (20ml), triethylamine (5g, 495mmol) and hydroxylamine hydrochloride (10g, 143mmol), stirred under reflux for 6.5 hours and cooled to 10°C in an ice bath Next, slowly adjust the pH to 10 with concentrated ammonia water, and solids precipitated out. Continue cooling in an ice bath for 1 hour to crystallize, filter with suction, and dry the filter cake to obtain 10.7 g of white solids, yield 95.2, mp: 114-118°C.
Embodiment 2
[0034] Preparation of 0-p-toluenesulfonyl-3-acetylpyridinium oxime (5):
[0035] 10.3g (75mmol) of 3-acetylpyridine oxime (4) and 36mL of anhydrous pyridine were mixed uniformly under stirring, cooled in an ice bath, 15.7g (82mmol) of p-toluenesulfonyl chloride was added in batches, and the temperature was controlled below 5°C. Then stirred overnight at 0°C, poured into 400g of crushed ice, and filtered to obtain 19.3g of a yellowish solid, yield 88.6%, mp: 77-79°C.
Embodiment 3
[0037] Preparation of 2-amino-1-(3-pyridyl)ethanone dihydrochloride (6):
[0038] Under nitrogen protection, potassium ethylate (6.4g, 76mmol) was added to absolute ethanol (50ml), and after the internal temperature dropped to room temperature, 40mL of 0-p-toluenesulfonyl-3-acetylpyridinium oxime (5) (20g, 68.1mmol) was slowly added ) ethanol suspension, stirred vigorously, a large amount of orange-yellow solid was precipitated, the reaction solution became thicker and thicker, the inner temperature rose to 50°C and then dropped to room temperature for 4 hours of reaction, adding anhydrous diethyl ether (400mL), filtered, and the filter cake was washed with Wash with water and ether, add concentrated HCl (100mL) slowly to the filtrate and lotion, a large amount of orange precipitate precipitates, cool in an ice bath for 1 hour and filter, dry the filter cake and recrystallize with absolute ethanol to obtain 10.5g off-white crystals , yield 78.5%, mp: 171-173°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com