Antibiotic polyurethane material and preparation method thereof
A polyurethane material and polyurethane technology, applied in the field of preparation of antibacterial polyurethane materials, can solve problems such as lack of antibacterial effect, achieve long-term antibacterial effect, easy to realize, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
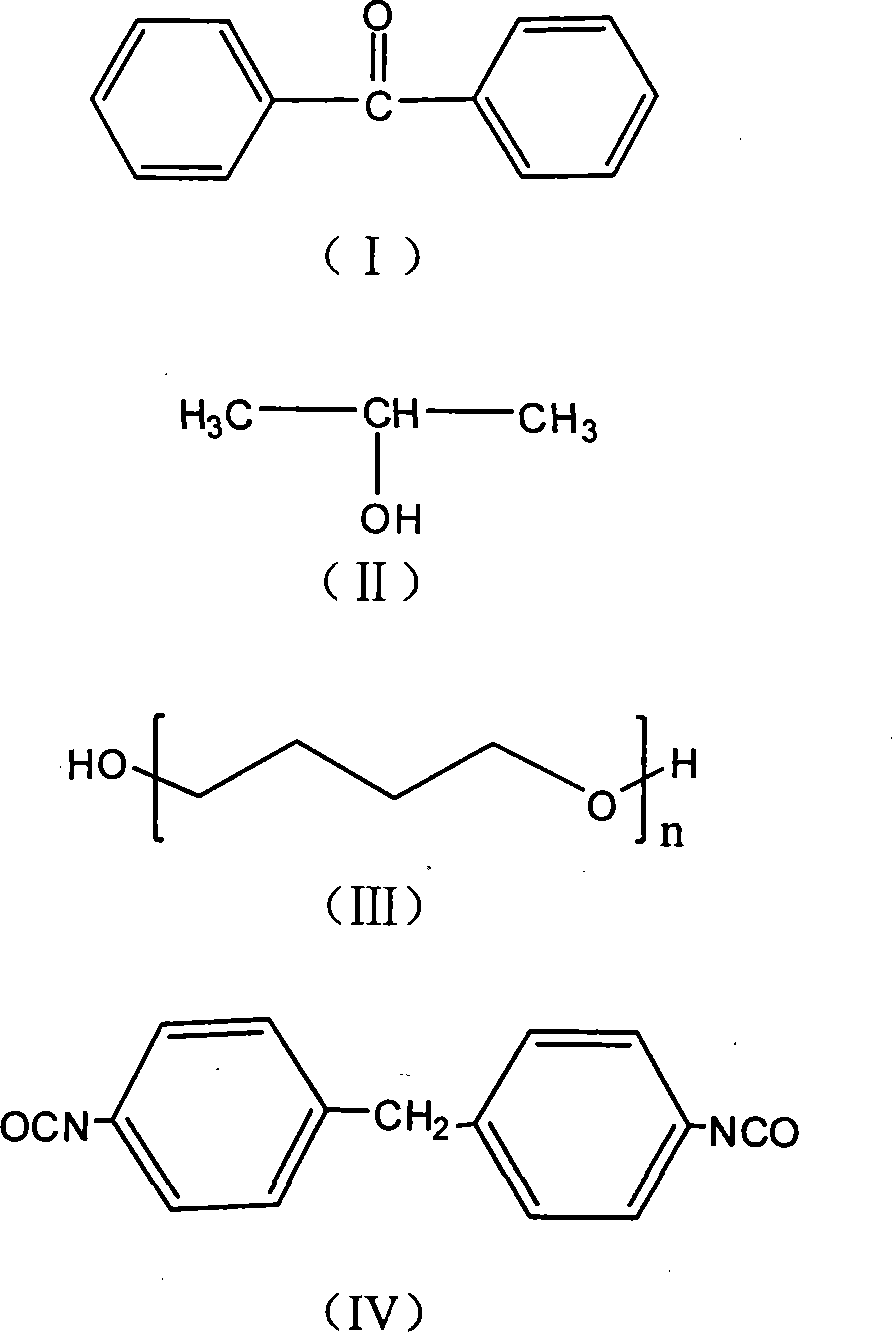
[0035] Step 1): Benzophenone (0.1mol; 18.20g) and isopropanol (0.5mol) were mixed first, then acetic acid (0.005mol; 0.03g) was added, and the mixture was charged into a 500mL round bottom flask, At a temperature of 25° C., exposed to 365 nm ultraviolet light for reaction, the product (1,1,2,2-tetraphenylethylene glycol) was precipitated, and the precipitated product was purified by recrystallization from acetic acid.
[0036] obtained pure 1 H-NMR (CDCl 3 ): δ=3.05 (OH, 2H), 7.00-7.50 (phenyl, 20H).
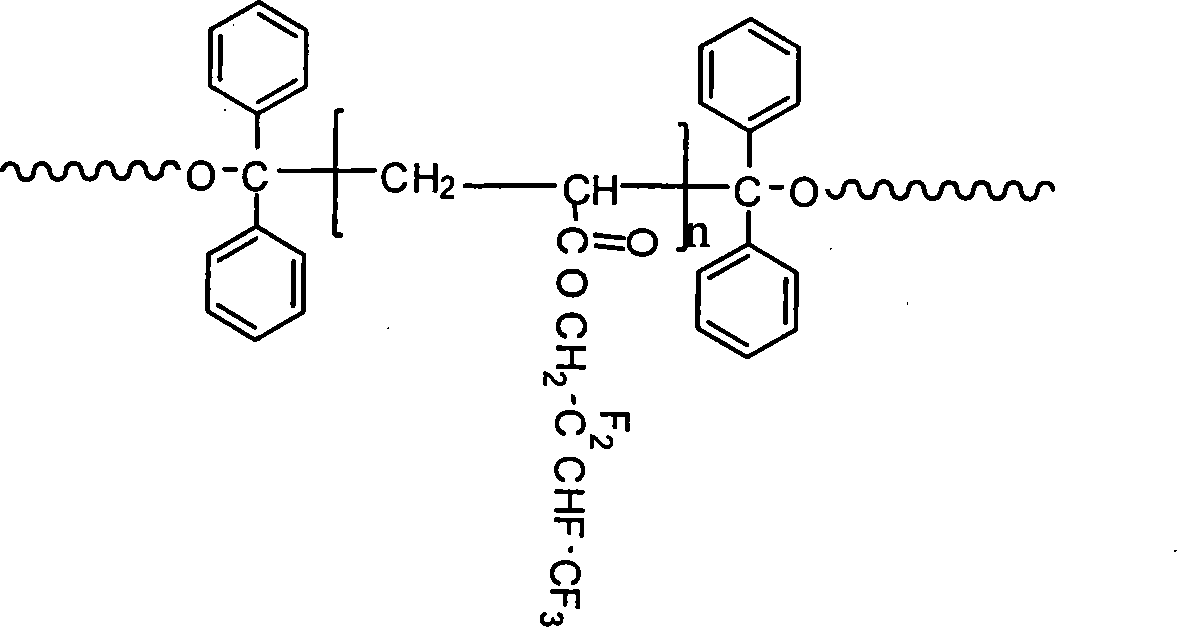
[0037] Step 2): Mix polytetrahydrofuran ether diol (0.01mol; 10.00g) and 4,4'-diphenylmethane diisocyanate (0.02mol; 5.00g) into 50-100mL tetrahydrofuran, and the mixture is loaded into a 500mL In a three-necked round-bottomed flask, under nitrogen protection, stir and mix at 60-85°C for 1-4 hours to obtain a prepolymer; when the temperature drops to 20-45°C, add 1,1,2,2-tetraphenylethylene glycol ( 0.01mol; 3.66g), adding the molar ratio is: 1,1,2,2-tetraphenylethylene glyco...
Embodiment 2
[0042] Step 1): First mix benzophenone (0.1mol; 18.20g) and 2-propanol (0.5mol), then add glacial acetic acid (0.005mol; 0.03g), and the mixture is charged into a 500mL round bottom flask , at a temperature of 30° C., exposed to 365 nm ultraviolet light for reaction, the product (1,1,2,2-tetraphenylethylene glycol) was precipitated, and the precipitated product was purified by acetic acid recrystallization.
[0043] obtained pure 1 H-NMR (CDCl 3 ): δ=3.05 (OH, 2H), 7.00-7.50 (phenyl, 20H).
[0044] Step 2): Mix polycarbonate diol (0.01mol; 20.00g) and 4,4'-diphenylmethane diisocyanate (0.02mol; 5.00g) into 50-100mL butanone, and the mixture is charged to 500mL In a three-necked round-bottomed flask, under the protection of nitrogen, stir and mix at 60-85°C for 3 hours to obtain a prepolymer; when the temperature drops to 35°C, add 1,1,2,2-tetraphenylethylene glycol (0.01mol; 3.66g), the molar ratio of adding is: 1,1,2,2-tetraphenylethylene glycol: diisocyanate=0.3:1~1:2, an...
Embodiment 3
[0049] Step 1): First mix benzophenone (0.05mol; 9.10g) and isopropanol (0.25mol), then add 1mL of glacial acetic acid, and the mixture is charged into a 500mL round bottom flask, at a temperature of 25°C , exposed to 365nm ultraviolet light, the product (1,1,2,2-tetraphenylethylene glycol) was precipitated, and the precipitated product was purified by recrystallization from acetic acid.
[0050] obtained pure 1 H-NMR (CDCl 3 ): δ=3.05 (OH, 2H), 7.00-7.50 (phenyl, 20H).
[0051] Step 2): Mix polytetrahydrofuran ether diol (0.01mol; 10.00g) and 4,4'-diphenylmethane diisocyanate (0.02mol; 5.00g) and add 50-100mL N,N-dimethylacetamide , the mixture was put into a 500mL three-necked round-bottomed flask, stirred and mixed at 85°C for 1-4 hours under the protection of nitrogen to obtain a prepolymer; the temperature was lowered to 30°C, and 1,1,2,2-tetraphenyl Ethylene glycol (0.01mol; 3.66g), added in a molar ratio of: 1,1,2,2-tetraphenylethylene glycol: diisocyanate = 0.3:1 ~ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com