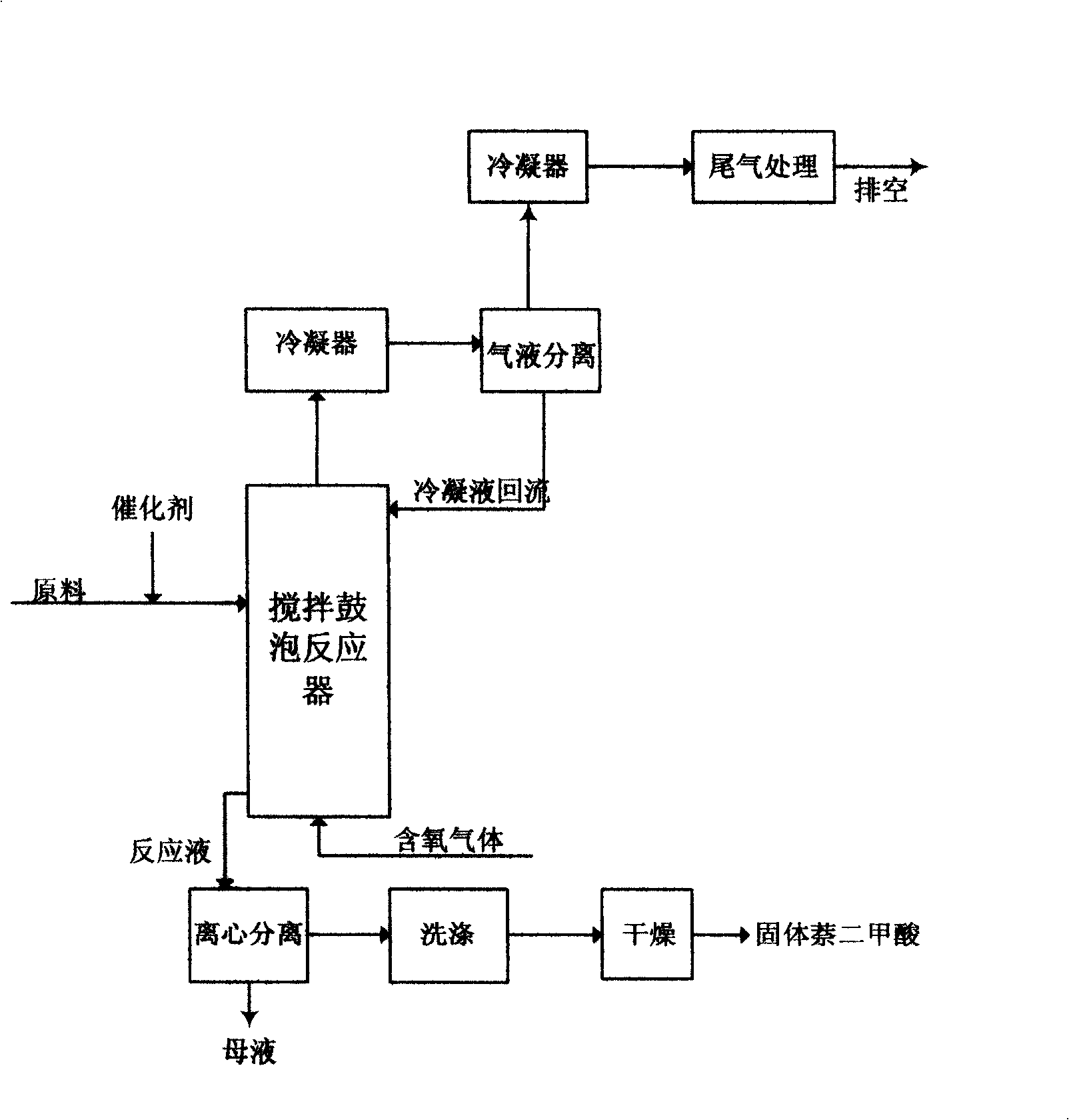
Process for producing 2,6-naphthalenedicarboxylic acid
A technology of naphthalene dicarboxylic acid and aliphatic carboxylic acid, applied in the field of compound preparation, can solve problems such as failure to achieve, and achieve the effects of reducing consumption, reducing production, and avoiding transitional oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 150g glacial acetic acid ((Co+Mn) / solvent is 0.016), 4.98g cobalt acetate tetrahydrate, 4.91g manganese acetate tetrahydrate (Co / Mn molar ratio is 1), 7.14g potassium bromide (Br / Co molar ratio For 3), 5.90g of potassium acetate (cocatalyst / (Co+Mn) molar ratio is 3) is added in the titanium reactor (the molar ratio of the solvent to the whole system material is 0.85), first use N 2 After exhausting the air in the kettle, pressurize to 2.4MPa, stir and heat to 200°C at the same time, and the pressure is 3.0MPa. Increase stirring speed to 800r / min, continuously supply compressed air with 3.0Nl / min, 2,6-diisopropylnaphthalene 44.67g ((Co+Mn) / 2,6-DIPN molar ratio is 0.19) in 4 hours Continuously feed in, continue to oxidize for 1 hour after the end of feeding to stop the reaction (the molar ratio of the total amount of oxygen introduced to 2,6-diisopropylnaphthalene is 40). After cooling and pressure relief, the reaction liquid was released, subjected to solid-liquid separ...
Embodiment 2
[0026] With 112.5g glacial acetic acid ((Co+Mn) / solvent molar ratio is 0.022), 37.5g propionic acid (glacial acetic acid: propionic acid weight ratio is 3), 6.73g cobalt acetate tetrahydrate, 6.62g manganese acetate tetrahydrate, 6.43 g potassium bromide (Br / Co molar ratio is 2), 5.30g potassium acetate (co-catalyst / (Co+Mn) molar ratio is 2) is added in the titanium material reactor (the molar ratio of the solvent to the whole system material is 0.86) , use N first 2 After exhausting the air in the kettle, pressurize to 2.4MPa, stir and heat to 200°C at the same time, and the pressure is 3.0MPa. Increase the stirring speed to 900r / min, continuously supply compressed air at 4.0Nl / min, and continuously supply 40.17g of 2,6-diisopropylnaphthalene dissolved in chlorobenzene within four hours, and continue to oxidize for one hour after the supply is completed. Reaction (the molar ratio of the total oxygen feed to 2,6-diisopropylnaphthalene is 53.3). After cooling and pressure rel...
Embodiment 3
[0030] Add 150g of glacial acetic acid ((Co+Mn) / solvent molar ratio is 0.022), 6.73g of cobalt acetate tetrahydrate, 6.62g of manganese acetate tetrahydrate, 6.43g of potassium bromide, and 5.30g of potassium acetate into the titanium reactor. use N 2 After exhausting the air in the kettle, pressurize to 2.4MPa, stir and heat to 200°C at the same time, and the pressure is 3.0MPa. Increase stirring speed to 900r / min, continuously supply the compressed air that is mixed with 20% (volume) carbon dioxide with 3.0Nl / min, 2,6-diisopropyl naphthalene 40.34g is continuously supplied in four hours, continue after supplying Oxidation for one hour stopped the reaction. After cooling and depressurization, the reaction liquid was released and subjected to solid-liquid separation, washed with glacial acetic acid and hot distilled water, and dried to obtain 35.07 g of earthy white solid powder. The purity and yield of 2,6-naphthalene dicarboxylic acid were analyzed to be 96.9%, respectively...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com