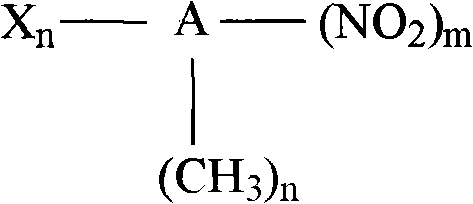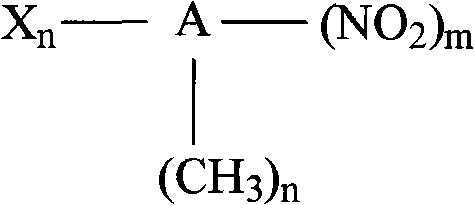Method for preparing arylamines compounds by arene nitro compound catalysis hydrogenation in H2O-CO2 system
A H2O-CO2, nitro compound technology, applied in the preparation of amino compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of reduced solubility, inflammable and explosive, insoluble in water, etc., and meet the reduction requirements. , the effect of eliminating pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Nitrobenzene hydrogenation prepares aniline
[0022] Add 10ml H into a 50ml stainless steel reaction kettle 2 O, 2ml nitrobenzene, 100mg reduced Ni / Al 2 o 3 -I catalyst, Ni / Al 2 o 3 -I is prepared by co-precipitation method, and the mass content of Ni is 40%. Tighten the reactor, purging with high-purity nitrogen for 3 minutes at room temperature, and then using 2MPa H 2 Replace 2 times to get rid of the air in the reactor. The reaction kettle was preheated in a constant temperature water bath at 50°C for 20 minutes, and then 6MPaH2 , 0.8MPa CO 2 , start stirring, and react at 50° C. for 30 min to obtain aniline. After extraction with benzene, it was analyzed by gas chromatography. The conversion rate of nitrobenzene was 55.7%, and the aniline selectivity was 99.1%.
Embodiment 2
[0023] Embodiment 2 Nitrobenzene hydrogenation prepares aniline
[0024] CO in the reaction system 2 Pressure is 3MPa, and other conditions are with embodiment 1. The conversion rate of nitrobenzene was 63.3%, and the aniline selectivity was 99.5%. After 75 minutes of reaction, the conversion rate of nitrobenzene was 99.5%, and the aniline selectivity was 100%.
Embodiment 3
[0025] Embodiment 3 Nitrobenzene hydrogenation prepares aniline
[0026] CO 2 Pressure is 11MPa, and other conditions are with embodiment 1. The conversion rate of nitrobenzene is 71.5%, and the selectivity of aniline is 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com